Patients and samples
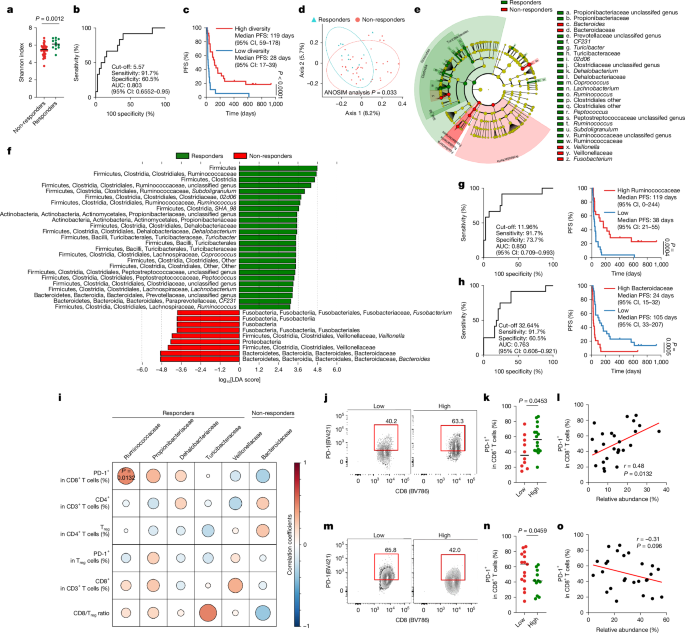
Patients with advanced NSCLC (n = 22) and patients with GC (n = 49) who received PD-1 blockade monotherapy (nivolumab or pembrolizumab) from March 2017 to September 2018 were enrolled in this study. Patients were separated into two independent cohorts in this study: the discovery cohort, in which patients with NSCLC or GC received PD-1 blockade monotherapy from March 2017 to December 2017 (n = 50; NSCLC, n = 15; GC, n = 35), and the validation cohort, in which patients with NSCLC or GC received PD-1 blockade monotherapy from January 2018 to September 2018 (n = 21; NSCLC, n = 7; GC, n = 14). Patients with HNSCC (n = 16) who received PD-1 blockade monotherapy (nivolumab or pembrolizumab) from June 2022 to October 2023 were also enrolled in this study. Patients with HNSCC who tested positive for human papillomavirus were excluded. In the MONSTAR cohort47, patients who received PD-1 blockade monotherapy (nivolumab or pembrolizumab) were enrolled from July 2019 to February 2022. Cancer types with n > 15 patients (advanced MM (n = 16), renal cell carcinoma (n = 44), GC (n = 32) and oesophageal cancer (n = 29)) were analysed in this study. Patients who were treated with antibiotics or microbiome intervention therapy within 1 month (the NSCLC and GC cohort and the HNSCC cohort) or 3 months (MONSTAR cohort) before the initiation of treatment were excluded from this study. Patients who achieved a complete or partial response or stable disease lasting >6 months were classified as responders and those who progressed on therapy or had stable disease <6 months were classified as non-responders per the Response Evaluation Criteria in Solid Tumors (RECIST; v.1.1). Forty-seven patients (discovery cohort: NSCLC, n = 4 and GC, n = 22; validation cohort: NSCLC, n = 7 and GC, n = 14) had an available quantity of TIL isolated; these samples were subjected to multiple immunological analyses. Stool samples were collected within 1 week before initial treatment with a DNA stabilizer (FS-0009, TechnoSuruga Laboratory) according to the manufacturer’s instructions and then stored at −80 °C. Fresh tumour samples were obtained from primary or metastatic tumours by needle or endoscopic biopsy within 2 weeks before initial administration and subjected to immunological analyses. Clinical information of patients was obtained from their medical records.
PD-L1 immunohistochemistry
Anti-PD-L1 monoclonal antibody (22C3, Dako; SP142 or SP263, Roche) was used for immunohistochemistry (IHC) with an automatic staining instrument (BenchMark ULTRA, Roche) as previously described54. PD-L1 positivity was defined as staining in 1% or more of the tumour cells.
Evaluation of mismatch repair status
Anti-mutL homologue 1 (MLH1; ES05) monoclonal antibody, anti-mutS homologue 2 monoclonal antibody (MSH2; FE11), anti-postmeiotic segregation increased 2 monoclonal antibody (PMS2; EP51) and anti-mutS homologue 6 monoclonal antibody (MSH6; EP49) (all from Dako) were used for IHC. Tumours were considered negative for MLH1, MSH2, PMS2 or MSH6 expression if there was a complete absence of nuclear staining in tumour cells. Normal epithelial cells and lymphocytes were used as internal controls. Tumours lacking MLH1, MSH2, PMS2 or MSH6 expression were considered deficient in mismatch repair, whereas tumours that expressed all these markers were considered proficient in mismatch repair.
Evaluation of Epstein–Barr virus status
Chromogenic in situ hybridization for RNA encoding Epstein–Barr virus was performed with fluorescein-labelled oligonucleotide probes (INFORM EBER probe) with enzymatic digestion (ISH protease 3, Roche) and an iViewBlue detection kit (Roche) with the BenchMark ULTRA staining system.
Analysis of human TILs
Tumour tissues were minced and treated within 72 h after surgery according to a TIL preparation protocol using an optimized tissue preservation reagent (Tumor & Tissue Preservation Reagent (TTPR)) and a TIL isolation reagent (Tumor & Tissue Dissociation Reagent (TTDR)), which were codeveloped by BD Biosciences55. Alternatively, tumour tissues were treated immediately with a gentleMACS Dissociator (Miltenyi Biotec) as previously described56. These samples were analysed using a BD LSRFortessa X-20 (BD Biosciences) or a FACSymphony system (BD Biosciences).
Flow cytometry analysis
Cells were washed with PBS containing 2% FCS and stained with the indicated antibodies and fixable viability dye. After staining for cell surface markers, the cells were intracellularly stained with antibodies with a FOXP3 staining buffer set (Thermo Fisher Scientific) according to the manufacturer’s instructions. After washing, cells were analysed with a BD LSRFortessa X-20 (BD Biosciences) or a FACSymphony system (BD Biosciences). The data were analysed with FlowJo software (v.10.8.1; BD Biosciences). The staining antibodies were diluted according to the manufacturer’s instructions. To analyse the similarity of the CD8+ TCR Vβ repertoires, the expression of TCR Vβ was evaluated and presented as an ordination plot by uniform manifold approximation and projection. To analyse the diversity of TLR expression on DCs, the Shannon index of TLR expression was calculated based on the expression of TLR1–TLR9.
DNA extraction from faecal samples
Total DNA extraction was conducted using a QIAamp DNA Stool Mini kit (Qiagen) according to the manufacturer’s instructions. To increase the recovery of bacterial DNA, particularly from Gram-positive bacteria, pretreatment with lytic enzymes was performed before extraction using a stool kit. In brief, 100 mg faecal sample was suspended in 10 ml Tris-EDTA buffer (pH 7.5), and then 50 μl of 100 mg ml–1 lysozyme type VI purified from chicken egg white (MP Biomedicals) and 50 μl of 1 mg ml–1 purified achromopeptidase (Fujifilm Wako Pure Chemical) were added. The solution was incubated at 37 °C for 1 h with mixing. Next, 0.12 g SDS (final concentration of 1%) was added, and the suspension was mixed until it became clear. Next, 100 μl of 20 mg ml–1 proteinase K (Fujifilm Wako Pure Chemical) was added, followed by incubation at 55 °C for 1 h with mixing. The cell lysate was then subjected to ethanol precipitation. The precipitate was dissolved in 1.6 ml ASL buffer from the stool kit and subsequently purified using a QIAamp DNA Stool Mini kit (Qiagen). For the HNSCC cohort, DNA was extracted using an ISOSPIN Fecal DNA kit (Nippon Gene) following protocol N as previously described57.
16S rRNA gene sequencing
Each library was prepared according to the ‘Illumina 16S Metagenomic Sequencing Library Preparation Guide’ with a primer set (27Fmod: 5′-AGR GTT TGA TCM TGG CTC AG-3′ and 338R: 5′-TGC TGC CTC CCG TAG GAG T-3′) targeting the V1–V2 region of the 16S rRNA gene. Next, 251-bp paired-end sequencing of the amplicon was performed using MiSeq (Illumina) with a MiSeq v.2 500 cycle kit. The raw sequences were demultiplexed and quality-trimmed with a FASTX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/index.html, v.0.0.14) and BBtrim (http://bbmap.sourceforge.net, v.33.21). A total of 20,000 reads per sample were randomly selected using seqtk (v.1.3-r106) for further analyses. The processed sequences were clustered into operational taxonomic units (OTUs) defined at a 97% similarity cut-off using UCLUST (v.1.2.22q). Representative sequences for each OTU were then classified taxonomically by using RDP Classifier (v.2.2) with the Greengenes (v.13 8) database. The bioinformatics pipeline QIIME (v.1.9.1) was used as the informatics environment for all relevant processing of raw sequencing data and the determination of relative bacterial abundances. For the HNSCC cohort, sequencing libraries of the V4 hypervariable region of the 16S rRNA gene were prepared as previously described58 and sequenced on an Illumina MiSeq instrument with V2 chemistry (2 × 251 bp reads).
Bacterial diversity analysis
Alpha diversity was estimated using the Shannon index, which captures both microbiota richness and evenness, after subsampling to an even depth (10,000 reads per sample). Beta diversity was assessed by unweighted UniFrac distance matrices and visualized by PCoA. The PCoA of unweighted UniFrac distances was performed with QIIME, and the results were plotted using R (v.4.02). ANOSIM was used to evaluate the significant differences between groups.
LEfSe
Kruskal–Wallis and pairwise Wilcoxon tests were performed, followed by LDA to assess the effect size of each differentially abundant taxon59. Bacteria with markedly increased abundance were defined as those with a log10[LDA score] > 3.
Estimation of YB328 phylotype abundance
Phylotype analysis, as delineated on the basis of the YB328 amplicon sequence variant (ASV) using 16S rRNA gene amplicon sequencing data, was performed for the following datasets: (1) prospective cohort of patients with HNSCC (V4 region, sequencing performed in this study); (2) MONSTAR cohort (V3V4 region, sequencing data obtained with permission from the MONSTAR alliance); and (3) two publicly available sequencing datasets48,60 (both V3V4 region). For ref. 60, data captured two healthy Japanese cohorts, namely the MORINAGA cohort (n = 704 for study accession DRP005906) and the NIBIOHN cohort (n = 1,280 for study accessions DRP007219, DRP007221, DRP007218, DRP007222 and DRP007220); the data were downloaded from the DDBJ Sequence Read Archive (DRA) in fastq format. For ref. 48, sequence data were obtained from the NCBI Sequence Read Archive (SRA) under BioProject PRJNA928744. In all cases, sequencing reads were denoised to obtain ASVs using DADA2 (v.1.26.0)61. For this purpose, primers in the reads were trimmed using Cutadapt (v.4.2)62, and read pairs without identifiable primers and undetermined bases (Ns) were discarded. Reads were then further trimmed by length (truncLen option) and filtered on the basis of their expected error (maxEE option) using the filterAndTrim function of DADA2. For the publicly available sequencing data, forward and reverse reads were denoised on a per-sample basis using the dada function in DADA2 with the options err = NULL and selfConsist = TRUE. For the data generated in this study (that is, the prospective cohort of patients with HNSCC) and the MONSTAR cohort data, error models for the forward and reverse reads were generated using the learnErrors function in DADA2 on a per-sequencing run basis. After denoising using the dada function with the run-specific error models, ASV tables were merged using mergeSequenceTables function in DADA2 and chimeras were removed using the removeBimeraDenovo function with method = “consensus”. For all datasets, a single ASV that perfectly matched the 16S rRNA gene sequence of YB328 was retained and used to calculate the relative abundance of YB328 (phylotype). To verify the specificity of detection of YB328, the V4 and V3V4 ASVs of YB328, as extracted from its whole-genome sequence, were compared against the Greengenes2 database (release 2024.09; Supplementary Data 1).
Metagenomic sequencing
The shotgun sequencing library for each microbial sample was constructed using Illumina DNA Prep (Illumina). The Illumina library was converted to a library for DNBSEQ using an MGIEasy Universal Library Conversion kit (App-A, MGI). Sequencing was performed on a DNBSEQ-G400RS (MGI) in 150-bp paired-end mode. All procedures were performed according to the manufacturer’s instructions.
Whole-genome sequencing of bacteria
The genomic DNA of YB328 and P. vulgatus AE61 was extracted using an enzymatic lysis method with Qiagen Genomic-tip 100/G columns according to the manufacturer’s protocol. The complete genome sequence of strain YB328 and a high-quality draft genome of P. vulgatus AE61 were determined using PacBio HiFi (high-fidelity) reads (Supplementary Table 7). PacBio sequencing, including library preparation and library quality control, were performed by Bioengineering Lab. In brief, short DNA fragments were removed using a Short Read Eliminator kit (PacBio) and retained DNA was sheared using a Covaris instrument to a size of 10–20 kbp. Sequencing libraries were then prepared using a PacBio SMRTbell Prep kit 3.0 and a SMRTbell gDNA Sample Amplification kit and quantified using a QuantiFluor dsDNA system (Promega) and an Agilent HS Genomic DNA 50 kb kit (Agilent Technologies) for quantification and size distribution, respectively. The library was then treated with a Revio Polymerase kit (PacBio) and sequenced on a Revio instrument. HiFi reads (Q20) were generated using SMRT Link (v.13.0.0.207600) to remove overhang adapter sequences and to create consensus sequences based on the resultant subreads. Lima (v.2.9.0) and pbmarkdup (v.1.0.3) were used for removing ultra-low PCR adapter sequences and PCR duplex reads, respectively. The resulting reads were assembled using Flye (v.2.9.5) and specifying the parameters –genome-size 3 m –asm-coverage 40. A phylogenetic tree showing the phylogenetic placement of YB328 and related taxa was constructed based on 120 single-copy marker genes using GTDB-Tk (v.2.4.0) with GTDB release 220 reference data. Protein sequences of identified marker genes (using the identify function of GTDBTk) were aligned using the align function of GTDBTk with the option skip_trimming. Aligned protein sequences were then loaded into an ARB (v.7.0) database, representative genomes were selected and the data were exported with custom masks for uninformative sites. Approximate maximum-likelihood phylogenetic trees were inferred using FastTree (v.2.1.1053), and the trees were visualized in ARB. The average nucleotide identity (ANI) values were then calculated using fastANI63 through comparisons of YB328 and closely related species. Genome gene identification was performed with Prodigal (v.2.6.3) with the default settings64. The predicted protein sequences were further analysed by matching against the Clusters of Orthologous Groups (COGs) database (release 3.10 in the Conserved Domain Database)65 using RPS-Blast (BLAST v.2.9.0+; E value cut-off of 0.01), the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (release 2019-11-18)66 and the Virulence Factor Database (VFDB; release 2022.04)67 and by protein alignment using DIAMOND (v.2.0.13)68. Coding sequences unique to strain YB328 (see Extended Data Fig. 4a for genome accessions) were identified using CompareM (v.0.1.2; https://github.com/dparks1134/CompareM) with default settings.
Quantification of species abundances based on metagenome sequencing data
Taxonomic profiling using metagenome sequencing data was performed through read-level taxonomic assignment with Kraken2 (v.2.1.3)69, followed by estimation of relative abundances using Bracken (v.2.9)70. For this purpose, paired-end reads were processed using fastp (v.0.23.2 or v.0.24.0)71 with the following settings: –trim_tail1 1 –trim_tail2 1 –cut_right –cut_right_window_size 4 –cut_right_mean_quality 15 –qualified_quality_phred 15 –unqualified_percent_limit 40 –trim_poly_x –poly_x_min_len 10 –n_base_limit 0 –length_required 75. Reads were then filtered using NCBI’s BMTagger (v.3.101) to remove reads derived from the human genomic DNA. Taxonomic assignment of the reads was performed using Kraken2 against a customized version of the GTDB (v.220), with options –confidence 0.30 –use-names –paired. Species abundances were then estimated based on the Kraken2 output using Bracken, with options -r 150 -t 10.
To construct the custom GTDB-based database, we built Kraken2 and Bracken databases using all species-representative genomes in the GTDB (v.220; n = 113,104 total bacterial and archaeal genomes), after replacing the corresponding genomes of the species P. vulgatus (representative: GCF_000012825.1) and H. mulieris (representative: GCF_020687165.1) by the two genome sequences generated in this study. Furthermore, to reduce the potential of false-positive assignments, a representative human genome sequence (GRCh38) was also included in the database. Construction of the Kraken2 and Bracken databases largely followed the scheme implemented in Struo2 (ref. 72), with a default k-mer length of 35 and specifying a read length of 150 for the bracken-build command.
To investigate the abundance of YB328 on a global scale, we analysed 1,188 human faecal metagenomic datasets from NCBI’s SRA. In this study, we focused on samples from healthy individuals; these were identified on the basis of the metadata available in GMrepo2 (ref. 73) and HumGut74 by retaining entries marked as ‘D006262’ and ‘Healthy’ for GMrepo2 and HumGut, respectively. Data were retrieved in FASTQ format using the SRA Toolkit’s prefetch and fasterq-dump commands, and then uniformly processed and analysed using fastp and Kraken2 as described above for the in-house generated sequencing data.
Bacterial identification and culture
All bacterial strains were isolated from fresh human faecal samples. The collected faecal samples were transported under 100% CO2 using a deoxygenating agent (Mitsubishi Gas Chemical) to a vinyl anaerobic chamber (COY) with an atmosphere composed of N2, H2 and CO2 (8:1:1). The faecal samples were diluted with pre-reduced dilution buffer to 1.0 × 10−8 g ml–1 in a tenfold dilution series. Fifty microlitres of 1.0 × 10−6 g ml–1, 1.0 × 10−7 g ml–1 and 1.0 × 10−8 g ml–1 diluted faecal fluid was inoculated on Eggerth–Gagnon (EG) agar plates75 supplemented with 5% (v/v) horse blood and M98-5 agar plates76. After incubation for 48 h at 37 °C, every colony grown on the plates was picked and subcultured on new plates for purification and growth. The obtained strains were preserved in pre-reduced nutrient broth (BD Biosciences) supplemented with 10% glycerol at −80 °C, and a portion of the colony was used for bacterial taxon identification. For bacterial taxon identification, the 16S rRNA gene sequence of the strains was amplified using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), and almost-full-length 16S rRNA genes were sequenced with the universal primers using a previously described procedure77. The species of the strains were determined by NCBI BLAST searches if the similarity between the query and the results exceeded 99%. Bacteroides vulgatus AE61 (renamed P. vulgatus), Sellimonas intestinalis AE3, Clostridium colicanis AE66, Eggerthella lenta AE7, Ruminococcus torques AE30 and YB328 were isolated from EG agar plates. Erysipelatoclostridium ramosum AM28 was isolated using M98-5 agar plates. Ruminococcus albus JCM 14654T was obtained from the Japan Collection of Microorganisms (JCM) (RIKEN). Acutalibacter muris DSM 26090T, Neglectibacter timonensis DSM 102082T and Clostridium leptum DSM 753T were obtained from the German Collection of Microorganisms and Cell Cultures. A. muciniphila BAA-835T and B. longum BAA-999 were obtained from the American Type Culture Collection (ATCC). The culture method for the commercial bacteria was performed according to the manufacturer’s instructions. For inoculation of these bacterial strains into mice, the cells were collected using degassed GAM broth (Nissui) in an anaerobic chamber and were then suspended at a concentration of approximately 1.0 × 109 cells per ml in pre-reduced PBS containing 0.05% (w/v) l-cysteine·HCl·H2O or 15% (w/v) glycerol solution for storage. Until inoculation, the suspended bacterial strains were kept at −80 °C.
Cell culture
The mouse colon cancer cell line MC38 (ENH204, Kerafast) and the mammary carcinoma cell line EMT6 (CRL-2755, ATCC) were cultured in high-glucose DMEM (Fujifilm Wako Pure Chemical) supplemented with 10% FBS (Biosera) and 2 mM l-glutamine. Mouse B16F10 (CRL-6475, ATCC) and B16F10-OVA (stable transfectant derived from B16F10 cells, which were generated in house) melanoma cell lines were cultured in RPMI-1640 (Fujifilm Wako Pure Chemical) supplemented with 10% FBS (Biosera) and 2 mM l-glutamine. All cell lines are confirmed to be free of mycoplasma contamination. For the induction of BMDCs from bone marrow (BM), 4 × 106 BM cells were cultured in tissue-culture-treated 6-well plates in 4 ml complete medium (RPMI-1640 (Fujifilm Wako Pure Chemical) supplemented with 2 mM l-glutamine and 10% FBS) containing 20 ng ml–1 GM-CSF (Peprotech) as previously described78. Half of the medium was removed on day 2, and fresh complete medium containing 40 ng ml–1 GM-CSF was added. The culture medium was entirely discarded on day 3 and replaced with complete medium containing 20 ng ml–1 GM-CSF. For the bacterial stimulation experiments, the multiplicity of infection (m.o.i.) of bacteria used to infect BMDCs was 100 or indicated in each figure legend. For the TLR stimulation experiment, flagellin (25 ng ml–1, InvivoGen), R848 (25 ng ml–1, Selleck Chemicals) and/or ODN-1826 (250 ng ml–1, InvivoGen) were administered for 24 h in complete medium containing 20 ng ml–1 GM-CSF. CDPs were isolated from BM cells by cell sorting and cultured with FLT3L (100 ng ml–1, Peprotech) and IMDM (Merck) supplemented with 2 mM GlutaMax (Merck), 55 μM 2-mercaptoethanol (Merck), 25 mM HEPES (Merck), 1 mM sodium pyruvate (Merck) and 10% FBS. CDPs were identified as LIN−CD117intCD135+CD115+CD11c−MHCII− BM cells, which lacked the markers of other cell lineages, including CD19, B220, CD3, NK1.1 and TER-119 (refs. 79,80,81). Bone marrow-derived macrophages were induced from BM cells as previously described82 and cultured in DMEM containing 10 ng ml–1 M-CSF (Peprotech). THP-1-derived reporter cell lines expressing human TLRs (hTLR7, hTLR8 and hTLR9) were cultured according to the manufacturer’s instructions (InvivoGen). Cells were stimulated with YB328 for 24 h, and supernatants were collected for downstream analyses. For YB328 fractionation, YB328 was cultured with GAM broth (Nissui) at 37 °C for 16 h. Using the collected cells, the cell membrane fraction and cytoplasm fraction were prepared using the bead-beating method as previously described83,84. Using the culture supernatant, the membrane vesicle fraction and supernatant fraction (without membrane vesicles) were prepared by ultracentrifugation previously as described85,86. For the stimulation of fractions of YB328, each fraction was added to the culture medium at a concentration of 10% (v/v). The MC38, EMT6 and B16F10 cell lines were pre-authenticated by ATCC using short tandem repeat sequencing. OVA expression in B16F10-OVA cells was confirmed by flow cytometry. THP1-Dual™ hTLR7, THP1-Dual™ hTLR8 and THP1-Dual™ hTLR9 cells were not authenticated but were functionally validated for responsiveness to their respective TLR agonists, serving as positive controls.
Animal models
C57BL/6 mice and BALB/cJ mice (6–10 weeks old) were purchased from CLEA Japan. OT-I TCR transgenic mice87 (provided by W. R. Heath, University of Melbourne), Myd88−/− mice88, Tlr7−/−, Tlr9−/− and Tlr7–/–Tlr9−/− mice (Oriental Bio Service, provided by S. Akira, WPI Immunology Frontier Research Center, Osaka University), Batf3−/− mice89 (The Jackson Laboratory, provided by K. Murphy, Washington University School of Medicine) and Kikume Green—Red (KikGR) mice90 (RIKEN BioResource Research Center, provided by M. Tomura, Kyoto University Graduate School of Medicine) were used. GF mice were bred and maintained in the gnotobiotic facilities of the Central Institute for Experimental Animals or purchased from CLEA Japa or Sankyo Labo and maintained within the gnotobiotic facilities of the National Institute of Advanced Industrial Science and Technology. Other mice were housed in cages under SPF conditions, provided with standard food, given free access to hypochlorous weak-acid water and housed with a 12–12-h light–dark cycle with lights on at 8:00. The temperature was maintained at 22 °C (20–26 °C), and the humidity was 45% (40–60%). In tumour challenge experiments, syngeneic mice were subcutaneously inoculated with 1 × 106 MC38 colon cancer cells, 1 × 106 EMT6 mammary carcinoma cells, 5 × 105 B16F10 melanoma cells or 5 × 105 B16F10-OVA melanoma cells in a total of 100 μl of PBS. The mice were randomized into different groups, ensuring that the tumour size was similar across all groups before the initiation of treatment. Anti-PD-1 monoclonal antibody (200 μg per mouse; clone: RMP1-14; BioLegend), anti-CSF1R monoclonal antibody (100 μg per mouse; clone: AFS98; Bio X Cell) or isotype control antibody (clone RTK2758; BioLegend) was intraperitoneally administered 3 times at 3-day intervals. Tumour length and width were measured on the indicated days, and tumour size was calculated as V = ½ (tumour length × tumour width2). The immunological profile of the TME was analysed on days 13 or 15 after tumour inoculation. For TLR agonist injection, TLR agonists (0.5 μg flagellin, 5 μg R848 and/or 5 μg per ODN-1826 per mouse) were intraperitoneally administered. Mice were monitored twice a week and euthanized when the subcutaneous tumour diameter exceeded 20 mm. In some FMT and bacterial administration experiments, mice were preconditioned with antibiotics (ampicillin 500 mg l–1 (Merck), neomycin 500 mg l–1 (Merck), metronidazole 1 g l–1 (Merck) and vancomycin 500 mg l–1 (Merck)) dissolved in sterile drinking water. Mice received bacterial or faecal transplantation by oral gavage using feeding needles. For FMT, we were able to collect faecal samples sufficient for in vivo animal models after examining gut microbiota from a total of 6 patients—3 responders and 3 non-responders—and used the faecal samples from these patients for each independent experiment. Faecal samples (0.1 g) were dissolved in 10 ml PBS and administered to mice by oral gavage (100 μl per mouse). For the bacterial administration experiments, we prepared the bacterial stock at 108 cells per ml and administered it to mice by oral gavage (100 μl per mouse). Intestinal-cell-labelling experiments were performed as previously described90. In brief, the small intestine was drawn from the abdominal cavity and photoconverted by exposure to violet light (435 nm, 40–50 mW cm–2) for 90 s by laparotomy. Nontargeted regions were protected from light using aluminium foil. For adoptive transfer experiments, splenocytes were collected from OT-I TCR transgenic mice, and CD8+ T cells were purified with CD8a microbeads (Miltenyi Biotec). SPF mice pretreated with antibiotics were subcutaneously injected with B16F10-OVA cells, and the sorted CD8+ T cells (1 × 106 cells per mouse) were transferred by intravenous injection. No blinding was performed. Sample sizes for animal experiments were chosen according to preliminary pilot studies12,91,92, and are in line with standards in the field.
Isolation of mouse immune cells
To isolate TILs and lymphocytes from the lymph nodes, tissue samples were collected and minced into small pieces, followed by digestion with BD Horizon Dri TTDR reagent at room temperature for 20 min. After dissociation, the cell suspension was filtered through a 70-μm strainer and washed with PBS supplemented with 1% BSA. For intestinal tissues, small intestines (duodenum, jejunum and ilium) were excised. The intestinal contents were removed by washing with HBSS. Fat tissue was also removed. Peyer’s patches and mesenteric tissues were carefully removed and digested in BD Horizon Dri TTDR according to the manufacturer’s instructions. The intestines were further opened longitudinally and cut into 5-mm long segments. Tissues were digested using a Lamina Propria Dissociation kit (Miltenyi Biotec) according to the manufacturer’s instructions.
ELISAs
BMDCs were stimulated with the indicated bacteria for 12 h, and chemokines and cytokines were measured using ELISA kits according to the manufacturer’s instructions (CXCL9, Abcam; CXCL10, Thermo Fisher Scientific; Proteome Profiler Mouse Chemokine Array kit, R&D Systems; and IL12p70, Thermo Fisher Scientific). For profiling chemokine expression with a Proteome Profiler Mouse Chemokine Array kit, the membrane was visualized using an LAS-4000 instrument (GE Healthcare), and the raw imaging data were processed and analysed with ImageJ software (Fiji, v.2.0.0).
Features of YB328
The cell structure of YB328 was observed by scanning electron microscopy (S-4500; Hitachi) and transmission electron microscopy (H-7600; Hitachi) as previously described93,94. In brief, for scanning electron microscopy observation, YB328 cells were fixed with 2% glutaraldehyde, post-fixed with 1% osmium tetroxide and dehydrated through a graded ethanol series followed by 3-methylbutyl acetate. For transmission electron microscopy observation, the cells were fixed with 2.5% glutaraldehyde and then post-fixed with 1% osmium tetroxide. The fixed cells were suspended in 1% aqueous uranyl acetate. The biochemical properties of YB328 were evaluated using an API ZYM system (bioMérieux) according to the manufacturer’s instructions.
Confocal microscopy
CD8+ T cells from OT-I TCR transgenic mice were prestained with 1 mg ml–1 Hoechst 33342 (Dōjindo Laboratories) at 37 °C for 10 min, washed and then cultured with the indicated bacteria-stimulated DCs pulsed with OVA peptides. After 4 h of culture, the cells were collected. Bacteria were prestained with SYTO Green Fluorescent Nucleic Acid stains (Thermo Fisher Scientific) and cultured with BMDCs prestained with CellTracker Deep Red dye (Thermo Fisher Scientific). All staining procedures were performed according to the manufacturer’s instructions. After 15 min of culture, the cells were collected. Cells were fixed with 4% formaldehyde solution for 30 min, permeabilized with 0.5% Triton X-100 for 10 min and blocked with PBS supplemented with 10% BSA for 1 h. The fixed cells were stained with an anti-NFATC1 (Thermo Fisher Scientific) monoclonal antibody overnight and then with a DyLight 488 secondary antibody (Thermo Fisher Scientific). All the antibodies were diluted in 10% BSA in PBS. Coverslips were mounted with ProLong Diamond Antifade mountant (Thermo Fisher Scientific). Images were captured by fluorescence microscopy (Carl Zeiss LSM-880, Zeiss) and analysed using ZEN software (Zeiss).
Time-lapse microscopy
The DCs and T cells were prestained with 0.5 μM CellTracker Green CMFDA dye, 0.5 μM CellTracker Red CMTPX dye, 0.5 μM CellTracker Deep Red dye (Thermo Fisher Scientific) or 1 mg ml–1 Hoechst 33342 at 37 °C for 10 min. Bacteria were prestained with SYTO Green Fluorescent Nucleic Acid stains (Thermo Fisher Scientific) according to the manufacturer’s instructions. Prestained DCs (2 × 105) were mixed with prestained CD8+ T cells from OT-I TCR transgenic mice (1 × 105) or bacteria (m.o.i. = 100) and seeded into a culture dish. The videos were recorded using an A1R MP+ multiphoton microscope system (Nikon) or a fluorescence microscope (Carl Zeiss LSM-880, Zeiss). The raw imaging data were processed and analysed with ImageJ software (Fiji, v.2.0.0).
IVIS spectrum in vivo imaging
Mice were depilated by hair removal cream 1 week before observation using an IVIS spectrum imaging system (IVIS kinetics, PerkinElmer), and the iVid-neo diet (Oriental Bio) was applied 2 weeks before observation to reduce autofluorescence from the diet. After 4 days of photoconversion, in vivo fluorescence images were obtained (for KikG, excitation filter = 465 nm, emission filter = GFP; for KikR, excitation filter = 570 nm, emission filter = Cy5.5). Analysis was conducted using Living Image software (v.4.7.3, PerkinElmer).
RNA sequencing
Total RNA was extracted from BMDCs using a RNeasy Micro kit (Qiagen) and further processed with a SMART-Seq v4 Ultra Low Input RNA kit (Takara Bio). cDNA library preparation was conducted with a Nextera XT DNA Library Preparation kit (Illumina). The prepared RNA sequencing libraries were subjected to next-generation sequencing with a NovaSeq 6000 (Illumina) using 150-bp read lengths in paired-end mode. The reads generated for each RNA sample were analysed and compared using the Illumina DRAGEN Bio-IT Platform. Sequencing reads were aligned and annotated to the UCSC mouse reference genome (GRCm38.p6) from Gencode. The number of transcripts per kilobase million was calculated and used for downstream analyses. Expression levels across samples were normalized by z score transformation.
Quantitative PCR
To determine the gene expression of BMDCs, total RNA was extracted from BMDCs using a RNeasy Micro kit (Qiagen). cDNA synthesis was performed with SuperScript IV VILO master mix (Thermo Fisher Scientific). The TaqMan gene assay probe was purchased from Thermo Fisher Scientific (assay ID: Batf3/Mm01318273_m1; 18S rRNA/Hs03003631_g1). TaqMan Fast Advanced master mix (Thermo Fisher Scientific) was used to perform PCR, and the results were examined with a QuantStudio 7 Flex Real-Time PCR system (Thermo Fisher Scientific). The PCR conditions were as follows: 50 °C for 2 min, 95 °C for 20 s, and 40 cycles of 95 °C for 3 s and 60 °C for 30 s. Gene expression was normalized to that of the endogenous control gene 18S rRNA. Relative gene expression between groups was determined using the comparative threshold cycle method. YB328 and P. vulgatus were quantified in faeces and tumour tissues by real-time PCR with a primer set targeting the recA gene of YB328 (forward primer: 5′-CCTCTTGGACCTTGCCGAAA-3′; reverse primer: 5′-ATACGCGTGCCGTTATACGA-3′) and the 16S rRNA gene of P. vulgatus (5′-forward primer: CGGGCTTAAATTGCAGATGA; reverse primer: 5′-CATGCAGCACCTTCACAGAT-3′) as previously described95 using a QuantStudio 5 Real-Time PCR instrument. The reactions (20 µl) contained 1 × Power SYBR Green PCR master mix and 500 nM each of the forward and reverse primers, and the thermal cycling conditions were as follows: 95 °C for 1 min, 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 15 s. A standard curve was prepared using purified genomic DNA of YB328 and P. vulgatus.
Multiplex IHC staining
Formalin-fixed and paraffin-embedded blocks of tumour specimens were sliced into 4-μm-thick sections on adhesion microscope slides (Matsunami). The tissue slides were deparaffinized and rehydrated for multiplex IHC staining. Antigen retrieval and subsequent staining were performed using an Opal 7-Colour IHC kit (Akoya Biosciences) according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed with Prism software (v.8; GraphPad), SPSS (v.21.0; IBM) and R (v.4.02; R Foundation for Statistical Computing). Patient characteristics were compared between the two groups using Pearson’s chi-square test and Fisher’s exact test. ROC curves for continuous variables were created by plotting the true-positive rate against the false-positive rate at each threshold. The AUC shown in each plot summarizes the performance of continuous variables. The cut-off values of continuous variables were determined as the maximum sum of the sensitivity and specificity in ROC analyses. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. No statistical methods were used to predetermine sample size. Data were analysed for a normal distribution using the Shapiro-Wilk test before comparisons. The relationships between two groups were compared using a two-sided Student’s t-test for normally distributed data or the nonparametric Mann–Whitney U-test. For multiple testing, significance was determined by one-way ANOVA followed by Bonferroni’s correction. Tumour volume curves were compared using two-way ANOVA with the Tukey–Kramer method. The correlation coefficient was evaluated by Pearson’s correlation. Univariate or multivariate analyses were performed with the Cox regression model. All graphs of animal and in vitro experiments show representative data from at least two independent experiments. P < 0.05 was considered to indicate significance. NS, not significant.
Inclusion and ethics
This study was conducted in Japan with the involvement of local researchers throughout the research process, from conceptualization to data analyses and manuscript preparation. All patients provided written informed consent before sampling, according to the Declaration of Helsinki. Analyses of cohort samples were conducted in a blinded manner and approved by the National Cancer Center Ethics Committee (IRB protocol numbers: GC and NSCLC cohort: 2015-048 and 2017-007; HNSCC cohort: 2022-346; MONSTAR cohort: 2018-367). The GC and NSCLC cohort and the MONSTAR cohort are registered in the UMIN Clinical Trials Registry (https://www.umin.ac.jp/ctr/, IDs: UMIN000019129 and UMIN000036749, respectively). The study was not designed to evaluate outcomes based on participant characteristics such as sex or ethnicity. Animal care and experiments were conducted according to the guidelines of the animal committee of the National Cancer Center/Institutional Animal Experiment Committee of National Institute of Advanced Industrial Science and Technology after approval by the Ethics Review Committee for Animal Experimentation of the National Cancer Center (protocol numbers: K24-007 and K24-010) and the Institutional Animal Experiment Committee of National Institute of Advanced Industrial Science and Technology (protocol number: 2022-0413).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.