Statistics and reproducibility
All statistical tests were performed using GraphPad Prism 10, using either two-tailed Mann–Whitney U-tests or two-tailed t-tests, as specified in the legends. Tests for normality were performed when appropriate. Sample sizes were not predetermined. For all experiments, cells were randomized such that within a biological or technical replicate all cells were analysed equally with no sub-sampling. Microscopy imaging, western blotting and quantitative PCR with reverse transcription (RT–qPCR) were repeated independently at least three times with similar results. P values and the numbers of observations (n) are provided in the figure legends.
Cell culture and cell lines used in this study
All cells were cultured in a humidified chamber at 37 °C with 5% CO2 and 1% streptomycin and penicillin (Gibco, 15140122). HEK293T cells (ATCC), HEK293T cells tagged with UBTF–sfGFP, NPM1–mtagRFP and FBL–Halotag (gift from the Leonetti laboratory), HCT116 cells (gift from Y. Kang) and MCF7 cells (gift from Y. Kang) were cultured in DMEM (GIBCO, 11995065) supplied with 10% FBS (R&D Systems, S11150H). MCF10A (gift from Y. Kang) cells were cultured in DMEM/F12 medium (Thermo Fisher Scientific, 11320082) supplied with 5% horse serum (Sigma-Aldrich, H1138), 20 ng ml−1 EGF (Novoprotein, C029), 10 ng ml−1 insulin (Sigma-Aldrich, 91077 C) and 1 µg ml−1 hydrocortisone (Sigma-Aldrich, H0888). All cells were checked for Mycoplasma and authenticated. For imaging, cells were treated with trypsin (trypsin-EDTA 0.05%, Thermo Fisher Scientific, 25300054) for dissociation and then seeded into 96-well glass-bottom dishes (Cellvis, P96-1.5H-N) coated with bovine fibronectin (Sigma-Aldrich, F1141) diluted 1:4 in 1× DPBS (Thermo Fisher Scientific, 14190144). All rDNA plasmid transfection experiments were performed in HEK293T cells, and all other experiments were performed in MCF10A cells unless otherwise noted.
Recombinant human rDNA plasmid designs
A plasmid containing a previously described minimized 5′ ETS (mini 5′ ETS) that is compatible with human SSU biogenesis was used as a starting point (pSK_M323) for the design of plasmids described in this Article44 (Extended Data Fig. 7a). The structure of the human SSU processome in state pre-A1 (Protein Data Bank: 7MQ8) was used to redesign the 3′ and 5′ hinge RNA duplexes between U3 snoRNA and the 5′ ETS (Extended Data Fig. 7c,d). Nucleotide substitutions were introduced that maintain the overall nucleotide composition of the duplexes while only allowing matching variants to base pair (Extended Data Fig. 7e,f). Variants of 3′ and 5′ hinges of the 5′ ETS and U3 snoRNA were combined (pSK_M432-pSK_M435) by including variants of the complete human U3 gene upstream of the RNA polymerase I promoter, resulting in a bidirectional promoter for pre-rRNA (RNA polymerase I) and U3 snoRNA (RNA polymerase II) (Extended Data Fig. 7g). Plasmids coding for either wild-type or mutant SSU pre-rRNAs were generated by terminating transcripts after the first 48 nucleotides of the 5.8S gene and a plasmid coding for the LSU pre-rRNAs contained the first 53 nucleotides of the mini 5′ ETS followed by ITS1, 5.8S, ITS2, 28S and the 3′ ETS (Extended Data Fig. 7b). A probe for ITS2 was introduced from a previously published plasmid (pSK_M349) that was shown to give rise to mature human LSUs45.
Plasmid construction
FM5-Nop56-mcherry was a gift from D. W. Sanders. FM5-mTagBFP2-NPM1, FM5-RPA16-GFP and FM5-RPS6-Halotag lentiviral DNA plasmids were generated using the FM5 lentiviral vector (gift from D. W. Sanders)57. A DNA fragment encoding human RPS6 was amplified from original plasmids (DNASU Plasmid Repository, HsCD00043827) by PCR using the Q5 High-Fidelity 2X Master Mix (NEB) with oligonucleotides synthesized by IDT. gBlocks encoding RPA16 or RPS4X proteins were ordered from IDT. DNA fragments containing NPM1 and RPA16 were PCR amplified from gifted plasmids from J. A. Riback. The In-Fusion HD cloning kit (Takara) was used to insert the fragments into the desired linearized vector featuring a GS-linker–fluorescent tag fusion. All of the constructs were confirmed by Sanger sequencing (GENEWIZ).
IF analysis
Cells were fixed in 96 well glass bottom plates with 4% PFA for 10 min, washed with 1× PBS twice and then permeabilized with 1× PBST (with 0.5% Triton X-100) for 15 min. The samples were then blocked in 2% BSA in PBS for 30 min and then incubated with primary antibodies in 2% RNase-free BSA (VWR, 97061-420) for 2 h at 37 °C (a detailed antibody list is provided in Supplementary Table 1). Three 1× PBS washes were conducted for 5 min each. For non-conjugated antibodies, anti-mouse or anti-rabbit secondary antibodies with the desired fluorophores were used at a 1:1,000 dilution for 2 h at 37 °C. Three 1× PBS washes were conducted with 5 min each before imaging.
RNA-FISH
SABER FISH was performed as previously described38,61. Probes were designed across the ribosomal RNA sequence and additional hairpin sequences are appended at the 3′ end of the probe for primer exchange reaction concatemerization with hairpin_28 (paired with 488 fluorescent oligos for imaging) or hairpin_25 (paired with 647 fluorescent oligos for imaging). A list of all of the probe sequences is provided in Supplementary Table 1. Note that, because early cleavage sites are cleaved and degraded rapidly, we used FISH probes tiling 5′ ETS (upstream of 01 site) and 3′ ETS (downstream of 02 site) to improve detection. For probing ribosomal RNAs transcribed from synthetic plasmids, 18S* and 28S* probes were designed (Supplementary Table 1) to probe the unique (around 16–20 nucleotides) insertion within the 18S and 28S sequence from plasmids44. Moreover, an antisense 18S* probe was designed to ensure the RNA-FISH signal specifically comes from RNA instead of DNA (Extended Data Fig. 8c and Supplementary Table 1). To specifically probe endogenous ribosomal RNA, we used probes hybridized to parts of the 5′ ETS region that are excluded from the synthetic rDNA plasmids (Δ1,2,3)44. Moreover, as a unique sequence is inserted within 28S (28S*), we designed a FISH probe flanking the insertion, located upstream and downstream of 28S*, for selective hybridization to endogenous 28S rRNA (endogenous 28S in Supplementary Table 1). For analysing whether plasmid-derived RNAs are exported to the cytoplasm, we used the endogenous 28S probe for segmenting the nuclei and cytoplasm (details are provided in the the ‘Quantitative image analysis’ section). IF analysis was conducted after completing all of the FISH steps, starting from the blocking step as described in the ‘IF analysis’ section. Murine RNase inhibitors (NEB, M0314L) were used at a 1:200 dilution in all of the steps of IF to preserve the RNA-FISH signals.
Microscopy
A Nikon CSU-W1 SoRa spinning-disc confocal microscope equipped with a Yokogawa SoRa pixel reassignment-based super-resolution device was used for rapid super-resolution imaging. The system was built around the Nikon Ti2-E fully motorized microscope and is equipped with dual Hamamatsu Fusion BT sCMOS cameras. The W1 Sora system is equipped with 405, 488, 514, 532, 561, 594 and 640 nm laser lines. For this work, a Nikon CFI Plan Apo Lambda D ×60 oil (MRD71670) lens was used with a ×2.8 SoRa magnification and 405, 488, 561 and 640 nm lasers. A Mad City Labs piezo z stage was used for z-stack acquisition. All acquisitions were performed using the Fusion BT in Ultra Quiet readout using correlated double sampling. In some cases, Denoise.ai (Nikon software) was used for images shown and analysed in this study.
Lentiviral packaging and transduction
Lentiviruses were made using HEK293T cells seeded in a six-well plate at 70–80% confluence. The desired plasmids were transfected together with helper plasmids VSVG and PSP using Lipofectamine 3000 (Invitrogen, L3000008) according to previously described protocols57,62. Viruses were collected 48 h after transfection and filtered through syringe filters with a 0.45-µm pore size (VWR). Lentiviral transduction was conducted in 96-well plates at 30% cell confluency for 2 days and cells were then expanded to make stable expression lines and sorted using fluorescence-activated cell sorting for polyclonal lines, with a tight window for each fluorescent protein intensity.
Transient transfection
rDNA plasmids (including engineered mutant plasmids) were transfected into HEK293T cells (for one well of a 24-well plate, 600 ng plasmids were transfected into 150,000 HEK293T cells) using lipofectamine 3000 (Invitrogen). Unless otherwise stated, for all plasmid transfection experiments, cells were seeded into 96-well glass-bottom plates 24 h after transfection and fixed for SABER FISH 48 h after transfection. Specifically, all cytoplasmic export analyses were performed at 48 h after transfection (Figs. 3f–h and 4e,i and Extended Data Fig. 9e). This ensured that all plasmids were compared at the same time after transfection. For the wild-type and mutant SSU-only plasmid transfection experiments comparing nucleolar morphology and radial outflux in Fig. 4f–h and Extended Data Fig. 10c–h, we optimized the timing of transfection to be 24 h to achieve the optimal number of de novo SSU nucleoli.
Endogenous N-terminal tagging of NPM1 with mTagBFP2 using CRISPR–Cas9
Endogenous N-terminal tagging of NPM1 in MCF10A cells was performed as previously described63,64. An oligonucleotide pair encoding an NPM1-targeting gRNA (TGTCCATCGAATCTTCCAT) was cloned into a modified lentiCRISPRv2-puro plasmid (from A. Lin) through the BsmBI restriction site. MCF10A cells were transfected using the FuGENE HD transfection reagent (Promega, E2311) with plasmids containing the cloned gRNA and a donor plasmid. The donor plasmid was constructed by cloning the tag with a flexible linker flanked by 300 bp homology arms complementary to the N terminus of the NPM1 gene into the pUC19 vector (Thermo Fisher Scientific, SD0061). Then, 3 days after transfection, mTagBFP2-positive cells were single-cell sorted into 96-well plates. These single-cell clones were then cultured and expanded for tagging validation through western blotting and junction PCR of the specific genomic locus (Extended Data Fig. 2f,g) and imaging to confirm correct subcellular localization.
All ribosomal RNA processing perturbations used in this study
CX-5461 Pol I inhibition
Cells were treated with 10 μM final CX-5461 (MedChem Express, HY-13323) for 90 min before fixation and imaging. DMSO was used for the control group.
FVP broad rRNA processing inhibition
Cells were treated with FVP at a final concentration of 2 μM (MedChem Express; HY-10005). As a control, cells were treated with DMSO. For the 5eU imaging and 5eU–seq, cells were pretreated for 1 h to broadly inhibit processing prior to a 15 min pulse and subsequent 0–90 min chase. All pulse–chase medium contained 2 µM FVP to ensure that processing inhibition was maintained throughout the 5eU pulse–chase labelling. For imaging the reformation of the multiphase nucleolus after FVP washout, cells were treated with 2 μM FVP for 2 h and then washed twice quickly with 1× DPBS before imaging in regular medium. Videos were taken at 37 °C with 5% CO2, every 2 min after FVP removal for 90 min. For 5eU imaging after FVP washout, cells were treated with 2 μM FVP for 2 h and then washed twice quickly with 1× DPBS before a 15 min 5eU pulse followed by a 0–90 min chase.
RPL5 shRNA
For shRNA vector cloning into a lentiviral plasmid for expressing shRNAs with puromycin selection, RPL5 shRNA (GATGATAGTTCGTGTGACAAA) sequences and a negative control (GCTCTTAACTAACGTCACCTA) sequence were separately cloned into pLKO.1 TRC vector after digestion with AgeI and EcoRI. Lentiviruses were produced as described above and added to cells for 5eU–seq and imaging experiments with MCF10A cells at 30% confluency. After 1 day, virus-containing medium was removed and replaced with fresh medium including 5 µg ml−1 of puromycin. After selection for another 3–4 days, cells in the negative control well that were not treated with virus were dying as expected, indicating that selection was effective. Selection was terminated by replacing the puromycin medium with normal MCF10A medium. Cells were then split for 5eU sequencing, imaging or RNA extraction followed by RT–qPCR (a list of the primer sequences used is provided in Supplementary Table 1) 4–5 days after adding shRNA viruses.
U3/U8 snoRNA ASO
U3 and U8 snoRNA ASO treatment was performed as previously described40 with several adaptations. For 5eU–seq experiments, in each well of a 6-well plate, 1.5 μl of 40 μM (stock) ASO diluted in 125 μl Opti-MEM (Thermo Fisher Scientific, 31985062) was combined with 7.5 μl Lipofectamine RNAiMAX (Thermo Fisher Scientific, 13778075) diluted in 125 μl Opti-MEM. After a 30 min incubation at room temperature, 1.75 ml of a suspension containing 250,000 cells in antibiotic-free medium (see the ‘Cell culture and cell lines used in this study’ section) was added to each well. Cells were incubated for 1.5 days before analysis using 5eU–seq (Fig. 2b,d and Extended Data Fig. 3h,i,n,o). For imaging experiments, in each well of a 96-well plate coated with bovine fibronectin (Sigma-Aldrich, F1141) diluted 1:4 in 1× DPBS (Thermo Fisher Scientific, 14190144), 0.05 μl of 40 μM ASO diluted in 4.165 μl Opti-MEM was combined with 4.165 μl Lipofectamine RNAiMAX diluted in 0.25 μl Opti-MEM. After a 30 min incubation at room temperature, 100 μl of a suspension containing 8,000 cells in antibiotic-free medium was added to each well. For 5eU-imaging experiments, cells were incubated for 2.5 days before fixation (Fig. 2c and Extended Data Fig. 6e). For experiments monitoring the inversion morphology, cells were treated again with ASO after 2.5 days according to the protocol described above, then incubated for an additional 2 days before fixation (Fig. 2e,f). We also tested the morphology change at shorter times (at 8, 12, 24 and 48 h), and observed that FCs and DFCs start moving towards the edge of the nucleolus within 8–12 h, with the phenotype fully developing within 24–48 h (Extended Data Fig. 5h–j). All incubations were performed at 37 °C and 5% CO2. ASO sequences were published in a previous paper40 and are listed in Supplementary Table 1.
FBL siRNA
FBL siRNA treatment was performed as described in the U3 and U8 snoRNA ASO treatment protocol with the following modifications. For 5eU–seq experiments and western blotting, 2 μl of 20 μM FBL siRNA (Supplementary Table 1) or control siRNA (Thermo Fisher Scientific, 4390843) diluted in 250 μl Opti-MEM was combined with 6 μl Lipofectamine RNAiMAX diluted in 250 μl Opti-MEM. For imaging experiments, 0.066 μl of 20 μM FBL siRNA or control siRNA diluted in 8.33 μl Opti-MEM was combined with 8.33 μl Lipofectamine RNAiMAX diluted in 0.2 μl Opti-MEM. For all of the experiments, cells were treated again after 24 h with FBL siRNA or control siRNA. Cells were incubated for an additional 3 days before performing 5eU–seq, collection for western blotting or fixation for 5eU imaging.
Total RNA isolation
For each well of a six-well plate, total RNA was collected in 200 μl 1× Buffer RLT (QIAGEN, 79216) and isolated using the QIAGEN RNeasy Mini Kit (74104), followed by 1 h of DNase treatment using TURBO DNase (Thermo Fisher Scientific, AM2238). DNase-digested RNA was then further purified using the Zymo RNA Clean and Concentrator-25 kit (R1017).
RNA electrophoresis
The RNA integrity after isolation was analysed and the ratios of 28S to 18S rRNA in U3 and U8 snoRNA ASO- and SCR ASO-treated samples were assayed using the Agilent RNA High Sensitivity Assay (Agilent, 5067-5579) on the 4150 TapeStation system (Agilent Technologies) according to the manufacturer’s instructions.
RT–qPCR for validation of perturbation
U3 snoRNA, U8 snoRNA and RPL5 mRNA KD efficiency were assayed using RT–qPCR using the Luna Universal One-Step RT-qPCR Kit (NEB, E3005) according to the manufacturer’s instructions, except that each reaction was scaled to 60 μl to allow for four technical replicates (12.5 μl) per sample. U6 snoRNA served as the loading control. RT–qPCR was performed on the Applied Biosystems QuantStudio 3 Real-Time PCR System instrument (A28567). Primer sequences used are listed in Supplementary Table 1. All primers were synthesized by Integrated DNA Technologies (IDT). U3, U8 and U6 snoRNA absolute amounts were determined using standard curves prepared for each primer set. U3 and U8 KD efficiency was assayed by comparing the absolute amounts of U3 or U8 snoRNA normalized to the absolute amounts of U6 snoRNA, in U3 or U8 snoRNA ASO- versus SCR ASO-treated samples.
Polysome fractionation and analysis
HEK293T cells after 48 h of transfection with the wild-type rDNA plasmid, SSU-only rDNA plasmid or no transfection control were treated with 100 μg ml−1 cycloheximide (CHX) for 10 min at 37 °C with 5% CO2 and polysome fractionation was performed as described previously with minor modifications65. Cells were then lysed in 400 µl polysome lysis buffer (25 mM HEPES, pH 7.3, 150 mM NaCl, 15 mM MgCl2, 1% Triton X-100, 8% glycerol, 0.5% sodium deoxycholate, 100 μg ml−1 CHX, 1 mM DTT, RNase inhibitors (NEB, M0314L, 1:60 dilution) and DNase (Thermo Fisher Scientific, AM2239, 1:400)) by incubating on ice for 15 min, followed by two consecutive centrifugations at 800g for 5 min, one centrifugation at 8,000g for 5 min and one centrifugation at 20,000g for 5 min (all at 4 °C) to remove nuclei and mitochondria. A 50 µl portion of the resulting supernatant was set aside as the input, while the remainder was loaded onto a 10–50% (w/v) sucrose gradient prepared in polysome gradient buffer (25 mM HEPES, pH 7.3, 150 mM NaCl, 15 mM MgCl2, 100 μg ml−1 CHX and 1 mM DTT). Ultracentrifugation was performed at 40,000 rpm for 2.5 h at 4 °C in a SW41 Ti rotor (Beckman Coulter). After ultracentrifugation, gradients were fractionated using a piston gradient fractionator (Biocomp) with continuous monitoring of absorbance at 254 nm to visualize ribosomal profiles. The fractions corresponding to ‘monosomes (80S)’ and ‘polysomes (>2 ribosomes)’ were collected and pooled separately. RNA was extracted from each fraction using ethanol precipitation followed by proteinase K (NEB, P8107S) digestion for 1 h at 50 °C, and cleaned using the Zymo RNA Clean and Concentrator-5 kit (R1016). Then, 25 ng of isolated RNA from input, monosome or polysome fractions was put into the RT–qPCR reaction. RT–qPCR was performed as described in the previous section using primers for 18S* (plasmid rRNA) and 18S (endogenous rRNA) (Supplementary Table 1). For each fraction (monosome or polysome), we calculated the incorporation of plasmid-derived rRNA across all ribosomes by normalizing the plasmid rRNA (18S*) abundance to the total rRNA (18S) abundance. Standard curves were generated to normalize for primer efficiencies.
5eU imaging
The 5eU imaging protocol was modified from previous studies33. In brief, cells were seeded 1 day before at 40% confluency in 96-well glass-bottom plates. The volumes of all reagents were kept at 100 μl for each well of the 96-well plate. 5eU (Thermo Fisher Scientific, E10345) solution was prepared at 0.5 mM in cell culture medium and added to cells with medium removed from the well. Cells were then kept in an incubator (37 °C with 5% CO2) for 15 min (pulse). Next, 5eU containing medium was removed and quickly washed twice with 1× DPBS containing 10 mM (excess) uridine (Sigma-Aldrich, U6381-5G) to outcompete the leftover 5eU. The culture medium containing 10 mM uridine was then added to the well before incubating for different chase timepoints (0 to 120 min) at 37 °C with 5% CO2. Note that all solutions mentioned above were kept at 37 °C using a heat block to minimize temperature-induced effects on RNA transcription and processing. For wells with different chase times on the same 96-well plate, the starting time of the 5eU pulse was staggered so that all the chase timepoints ended at the same time. For fixation, 4% paraformaldehyde (PFA) diluted from 16% PFA (Thermo Fisher Scientific, PI28906) with 1× PBS was used for 15 min at room temperature. Fixed cells were then washed twice with 1× PBS and permeabilized with 1× PBST (1× PBS and 0.5% Triton X-100) for 15 min. For click chemistry, the Click-iT Plus Alexa Fluor 647 Picolyl Azide Toolkit (Thermo Fisher Scientific, C10643) was used according to the manufacturer’s instructions, with the exception of using AZDye 647 Picolyl Azide (Click Chemistry Tools, CCT-1300-1) instead of the azide from the kit. After 30 min of applying the click chemistry reaction cocktail, cells were washed once with 1× PBS before imaging. For combining IF with 5eU imaging, IF steps were performed as described above before click chemistry.
5eU–seq
All 5eU pulse–chase labelling experiments combined with sequencing were performed in MCF10A cells except for method validation described in Extended Data Fig. 1c–g, which was performed on HEK293T cells. Cells were seeded for 5eU pulse–chase labelling such that their confluency was about 50–60% at the time of collection. Cells were pulse labelled with 5eU (Jena Biosciences, CLK-N002-10) for 15 min at 37 °C with 5% CO2. Cells were then removed from the incubator and quickly washed twice with 1× DPBS to remove 5eU from the cells. Cells were then chased with medium containing 10 mM uridine (Sigma-Aldrich, U6381) to outcompete the leftover 5eU in cells over different chase timepoints. All solutions were kept at 37 °C using a heat block to minimize temperature-induced changes to the cells, which could impact RNA transcription and processing. After the given chase time, cells were collected in 1× buffer RLT (Qiagen, 79216) and frozen at −80 °C for RNA isolation. RNA isolation was performed using the Qiagen RNeasy Mini kit followed by DNase digestion (TURBO DNase, Invitrogen, AM2238) to remove genomic DNA. RNA concentrations were measured using the Qubit RNA BR (Thermo Fisher Scientific, Q10211) system and the RNA integrity was analysed on the Agilent RNA High Sensitivity (HS) Tapestation.
Total RNA (10–15 µg) isolated after 5eU pulse–chase was click-reacted with biotin picolyl azide (Click Chemistry Tools, 1167-25) as described previously32 with the following modifications. Capture of biotinylated RNA was performed using 20 μl Dynabeads MyOne Streptavidin C1 beads (Invitrogen, 65002) after click chemistry. Captures and washes were performed as previously described except for a modification to the three 5 min washes of captured material, which was changed to 75 °C in no-salt urea buffer (4 M urea, 10 mM HEPES, pH 7.5, 10 mM EDTA, 0.5% Triton X-100, 0.2% SDS, 0.1% Na-DOC). We found that three rounds of sequential captures (as described previously32) as well an optimized protocol introduced here performing washes at high temperature and in a buffer lacking salt were essential to reduce the background of highly abundant mature rRNA (Extended Data Fig. 1a–d). RNA-seq library preparation was performed as previously described66. 5eU–seq libraries were then sequenced on the NovaSeq 6000 (Illumina) system with paired-end reads (either 150 × 150 or 100 × 200). The samples that failed quality control, such as failed click reactions, streptavidin captures or library amplifications, were excluded from downstream analyses.
High-performance computing
The analyses presented in this Article were performed on computational resources managed and supported by Princeton Research Computing, a consortium of groups including the Princeton Institute for Computational Science and Engineering (PICSciE).
Computational analysis of 5eU–seq cleavage and 2′-O-methylation
Sequencing read analysis pipeline
A custom snakemake pipeline was used to perform alignments, 2′-O-methylation analysis and cleavage analysis (https://github.com/SoftLivingMatter/5eU-seq-pipelines). Sequencing reads were trimmed with Trimmomatic (v.0.39)67 to remove adaptor sequences and bases containing low quality scores. Trimmed reads were then aligned to the rDNA genome (GenBank: U13369.1) using STAR (v.2.7.11a)68. Reads were sorted and indexed using Samtools (v.1.9-4)69 and only uniquely mapped reads were kept for further analysis. The fraction of reads that are cleaved at a given site was calculated as the number of non-spanning reads divided by the total number of reads (spanning and non-spanning) at a given site (Extended Data Fig. 1b) using featureCounts (v.1.6.4)70 (Subread package). rRNA cleavage sites were annotated based on the positions described previously14. All rRNA cleavage sites used in this study were manually inspected to define the sites of cleavage based on where the 5′ or 3′ end of reads piled up, demarcating a precise cleavage site. Specifically, well-defined cleavage sites correspond to those that undergo endonuclease cleavage and can be mapped at a base-pair resolution using 5eU–seq (examples shown in Extended Data Fig. 1b). In cases in which endonucleolytic rRNA cleavage events are followed by gradual degradation, a broader window (several nucleotides) downstream of an annotated site was used (Supplementary Table 1).
Cleavage efficiency measurement
Cleavage efficiency, defined as 1 over the time to reach 50% cleavage (t50), was estimated by fitting the observed 5eU–seq fraction cleaved over time (Extended Data Fig. 3) to a sigmoidal 4PL curve using Prism 10 software. We then computed the normalized cleavage efficiency (Fig. 2b) by dividing each by its respective control sample. Two-tailed t-tests were performed to estimate the significance of each treatment cleavage efficiency relative to its respective control. We note that, because FVP treatment completely blocks almost all rRNA cleavage steps, it is not possible to accurately estimate the time to reach 50% cleavage, so statistics were not determined.
2-O-methylation analysis
To determine 18S and 28S ribosomal RNA 2′-O-methylation levels over time, we applied RiboMethSeq computational analysis methods34,71 to 5eU–seq sequencing reads. In brief, 2′-O-methylated nucleotides are substantially more resistant to alkaline hydrolysis compared to non 2′-O-methylated nucleotides, resulting in fewer RNA fragmentation events at these sites. To map the nucleotides where fragmentation occurred, sequencing adaptors are ligated to the 3′ ends of fragmented RNA and 3′ ends of cDNA. This enables the identification of 2′-O-methylation sites at the single-nucleotide resolution by performing end mapping of reads. Specifically, the 5′ ends of read 1 sequences, which correspond to the 3′ end of the fragmented RNA, are mapped to the reference rRNA sequence. Next, modification levels at a given position were estimated by calculating the number of 5′-end read counts at a given 2′-O-methylated site compared with that of their neighbouring nucleotides, defined as ScoreC (see below). Nucleotides that are 2′-O-methylated, due to their resistance to hydrolysis, have substantially fewer 5′ end read counts, and therefore appear as ‘dips’ or areas of low coverage in the end-mapping profile (Extended Data Fig. 1h,i).
We calculated ScoreC at known 2′-O-methylation sites42 using a weighted average of the 5′ end read counts in a ±2 nucleotide window, recommended previously72, around each site. Nucleotides in the ±1 and ±2 neighbouring positions were assigned weight contributions of 0.9 and 1, respectively. If a separate 2′-O-methylation site was found within the ±2 window around a site, the 5′ end read counts at the former were skipped and those at the immediately preceding nucleotide were used alternatively. For the calculation of ScoreC, nucleotide positions of 2′-O-methylation sites on 18S rRNA were converted to those on 47S rRNA (U13369.1 human ribosomal DNA) by adding +3,655 to the 18S 2′-O-methylation positions (except for Am1678, Cm1703 and Um1804, to which +3,657 was added). For sites on 28S rRNA, nucleotides were converted to 28S 2′-O-methylation positions on 47S rRNA by adding +7,924, +7,920, +7,912, +7,911 and +7,903 for Am398 to Am400, Gm1316 to Gm2876, Cm3701 to Gm3944, Gm4042 to Cm4054, and Gm4196 to Gm4637, respectively. For data visualization, negative ScoreC values were clipped to zero. The heat map in Extended Data Fig. 3d was generated in Prism using the heat map function.
Defining fast and slow 2′-O-methylation sites
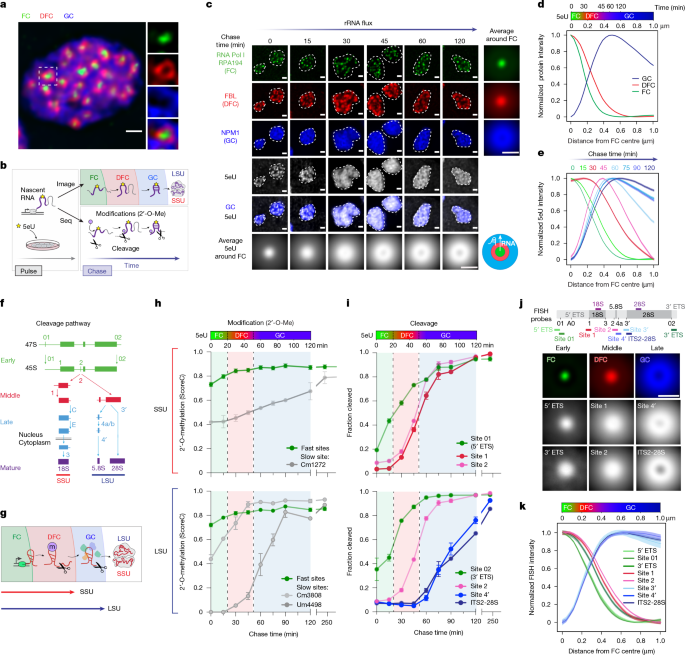
We measured the rates at which 2′-O-methylation occurs across all known 18S and 28S sites in our 5eU–seq data performed on MCF10A cells (n = 2 replicates) to determine which occur at a fast versus slow rate. 18S and 28S rRNA 2′-O-methylation sites that do not attain near steady state 2′-O-methylation levels within 30 min from transcription are defined as slow. All other 2′-O-methylation sites that meet this criterion and are defined as fast. Sites with high variation between replicates that made it challenging to confidently infer 2′-O-methylation rates were manually excluded. A table of high-confidence fast and slow 18S and 28S rRNA 2′-O-methylation sites is provided in Supplementary Table 1. We have provided examples of 5eU–seq data for individual high-confidence fast (Gm4494) and slow sites (18S-Cm1272, 28S-Cm3808, 28S-Um4498) in Extended Data Fig. 1i,j and Fig. 1h. The average of all high-confidence fast sites for each of 18S and 28S is shown in the SSU and LSU panels of Fig. 1h, respectively.
RiboMethSeq
RiboMethSeq for data in Extended Data Fig. 1g was conducted exactly as described previously34. For analysis, adapter sequences were trimmed from raw reads using Trimmomatic v0.39 with the following parameters: LEADING:30, TRAILING:30, SLIDINGWINDOW:4:15, AVGQUAL:30 and MINLEN:17. Quality control of the raw and trimmed reads was assessed using FastQC (v.0.11.9). Alignment to the reference rRNA sequence (18S: NR_003286.4; 5.8S: NR_003285.2; 28S: NR_003287.4) was done using Bowtie2 (v.2.3.5.1) with the default parameters. Sorting, indexing and extraction of mapped reads was done using Samtools (v.1.15.1), with the option -F 4 to exclude the unmapped reads. Subsequent analysis was conducted using R: the quantification of ribosomal RNA (rRNA) 2′-O-methylated residues was performed using the RNAmodR.RiboMethSeq package (v.1.18.0), the final processed data were exported using the writexl (v.1.5.0) package and display output was generated using Prism (v.10.3.0).
Pre-rRNA processing analysis by northern blotting
Total RNA was extracted from HEK293 cells labelled with or without 5eU with TRI reagent solution (AM9738, Thermo Fisher Scientific), according to the manufacturer’s instructions. Then, 5 µg total RNA was resolved on a 1.2% agarose/6% PFA denaturing gel, transferred overnight by capillarity onto a nylon Hybond N+ membrane (RPN203B, Cytiva), and hybridized with radioactively labelled probes (Supplementary Table 1). The probes were designed to detect all major pre-rRNA intermediates. The signal was acquired with a phosphorimager (FLA-7000, Fujifilm) and quantified using native multi-gauge software (v.3.1, Fujifilm).
Quantitative image analysis
All quantitative imaging measurements were performed using CellProfiler73 v.4.2.6. Cell segmentation was performed using cellpose74 v.2.3.2 and the RunCellpose plugin for cellprofiler. Full computational methods, pipelines and notebooks describing the calculation of the derived methods can be found at the GitHub repository (https://github.com/SoftLivingMatter/image-analysis-quinodoz-jiang-2024). To deploy cellprofiler plugins on HPC systems, a snakemake workflow75 wrapper was developed. It controls the resources and behaviour of each invocation and can be found at GitHub (https://github.com/softLivingMatter/snakemake-cellprofiler/).
Morphology
To quantitatively examine changes in cell morphology as a function of nucleolar perturbations, the following steps were performed in CellProfiler. First, the multichannel images were split into separate channels, such as GC, FC, DFC, FISH/5eU/other markers. The GC channel was used to define initial GC objects using minimum cross-entropy thresholding with a diameter range of 30–400 pixels, where each pixel corresponds to 0.0387 µm. Clumped objects were not separated as it produced too many falsely separated GC objects. As the images do not have a nuclear or cytoplasmic stain and some metrics are best measured across an entire cell, we computationally assigned nucleoli to the same cell based on if they were within 100 px to each other, producing a merged object mask. The mask was dilated by 50 px to measure metrics within the prospective nucleoplasm and find nucleolar features that may be outside the GC, such as FCs after FVP treatment, which causes detachment of the phases.
To find FC objects, the dilated GC objects were used to mask the FC image before performing ‘enhancing speckles’ with a feature size of 20 px using the Fast setting in the EnhanceOrSuppressFeatures module. The FC objects were found in the enhanced FC image using an adaptive, three-class Otsu threshold with the middle intensity class assigned to the background. This was selected to ensure that the identified FC objects were primarily in plane and in focus. The adaptive window was 100 px, clumped objects were separated by intensity and shape, and FC objects were selected with diameters of 7–50 px. As these FCs included objects within and outside of the GC, each FC was assigned to either a nucleolar or extra-nucleolar class using an overlap threshold of 50%. For example, an FC that overlaps an initial GC object by more than 50% was considered a nucleolar FC.
DFC objects were defined by masking the DFC image with the dilated GC objects. As the DFC phase organization is highly variable between treatments, the built-in IdentifyPrimaryObjects module was not able to perform adequately in all cases. Instead, DFCs were found by first thresholding with an adaptive, three-class otsu cut-off with a 150 px window. The middle class was assigned to background to ignore the diffuse DFC phase that develops after certain perturbations. The threshold image was converted to objects and then prospective DFCs were filtered to ensure they had an area of at least 20 px.
The GC, DFC and FC objects were combined to a single mask which was used as the support for measuring Pearson’s correlation and overlap of the image channels. The size and shape of each object set was also measured and used for scaling some metrics, discussed below. The intensity of the FC channel was measured in FC objects, and GC and probe (for example, FISH probe or 5eU) were measured in the GC and DFC phases. The distribution of FC and DFC intensity was measured over the initial GC objects using 20 scaled bins. A binary image of the initial GC objects was also measured to facilitate combining bins during post-processing. Each object was related to its corresponding dilated GC object before exporting to a csv for further analysis in Python.
The raw data produced by cellprofiler was further processed using jupyter notebooks and custom analysis scripts. Each object csv was read into a pandas dataframe and merged into a final result by the image and dilated GC unique identifier. Values such as total area or FC count were summed to provide a per-cell measurement. Average intensities per cell were calculated by first multiplying the mean intensity from cellprofiler with the object area, summing the result, then dividing by the total area per cell. Rim enrichment was calculated by summing the radial distribution fraction for the bins of interest and dividing by the fraction of the GC object over the same range of bins. Investigation of a range of rim widths found that the outer 20% of the GC rim provided the best discrimination between U3 KD and control cells. Circularity was estimated as 4 × π × area/perimeter2.
RDF estimation
To facilitate broader utilization of Radial distribution function (RDF) measurements, we developed a cellprofiler plugin, MeasureRDF, for calculating the radial distributions of object sets, such as the intensities of FISH or 5eU signals within a 1 µm radius from the FC centre, on a given input image. A separate plugin allows for further flexibility in performing upstream object finding and filtering and simplifies integration into other analysis tasks. In contrast to the MeasureObjectIntensityDistribution method available in cellprofiler, the MeasureRDF plugin uses a fixed distance in pixels and attempts to resolve overlapping objects as described below.
In the point-based measurement mode, the MeasureRDF plugin operates on a set of objects as the point sources along with a containing object. Here we used the GC boundary as the masking objects and FCs as the point sources. For each GC, the set of FCs are considered together. The image intensities are mean-centred with unit variance and pixel distances from each FC are determined. The RDF distribution is estimated by minimizing the difference between the scaled image intensity and a superposition of each FC point source with the same RDF. This allows for deconvolution of neighbouring point sources while providing an averaged estimate of the RDF. The estimated intensities are rescaled to the original intensity units before reporting to the user. In all of the plots, the intensity is either min–max scaled between the minimum and maximum intensities in the RDF or max scaled by dividing by the maximum intensities in the RDF to highlight intensity dynamics.
To visualize the location of each phase from the FC centre, the RDF distributions of FC, DFC and GC can be plotted on a colour bar, where the intensity of a phase is mapped to a given colour (FC, green; DFC, red; GC, blue). To identify the boundaries between the distinct phases, the intensities of each phase were subtracted from the intensity of their adjacent phase before plotting. Specifically, the distance at which the intensity of pairs of phases is equal is used to estimate the boundary between phases. Finally, the 5eU RDF distributions were used to map the distance the 5eU RNA has travelled from the FC centre to its corresponding chase time. For each chase time, the location of maximum EU intensity (5eU peak) was determined and used in a piecewise-linear interpolation between time and distance.
The MeasureRDF plugin can also operate in a boundary-measurement mode, which was used for the SSU-only and nucleolar periphery analyses performed in Fig. 4 and Extended Data Fig. 10a–f. In this setting, the source object boundaries are considered at r = 0, defined as the radial position of 50% DFC or GC normalized intensity, and the image intensity is measured as a function of distance from the boundary. The distance of each pixel to the object boundary is determined and used to estimate the RDF curve. In cases in which neighbouring point sources overlap, pixel intensities are assigned to the closest object.
Engineered plasmid measurements
For the images of de novo nucleoli, we manually classified individual nucleoli as endogenous, de novo or hybrid on the basis of their intensities for plasmid-expressed 18S*/28S* RNA and endogenous 5′ ETS rRNA. Specifically, endogenous nucleoli were those with high endogenous 5′ ETS RNA-FISH and no detectable plasmid-expressed 18S*/28S* RNA-FISH signal. Conversely, de novo nucleoli were those with high 18S*/28S* plasmid RNA-FISH signal, and no detectable endogenous 5′ ETS signal. Hybrid nucleoli contained FISH signals for both channels. Figure 3d demonstrates the range of nucleolar intensities of 5′ ETS and plasmid-expressed FISH intensities observed before further manual classification, where only high-confidence de novo versus endogenous nucleoli were analysed and any ambiguous nucleoli were excluded.
As the SSU-only rDNA plasmid produced nucleoli without detectable NPM1 intensity, a separate workflow was required to evaluate a subset of the morphology measurements described above. First, each channel was background corrected by subtracting the bottom 5 percentile value of each image. Next, the DFC (NOP56 or FBL) channel was blurred with a 5-px sigma Gaussian filter, which was found to produce better segmentation of SSU-only nucleoli. The blurred DFC image was used to detect nucleoli with a two-class global Otsu threshold and diameters between 10 and 150 px without declumping. The initial nucleoli objects were dilated by 10 px to act as support for the rim-based RDF measurement. Each channel’s intensity was measured in the nucleoli and the 10 -px rim as well as the object size and shape. For quality control, we manually checked the segmented SSU-only nucleoli objects and excluded those that were out of focus or incorrectly segmented.
To measure cytoplasmic and nuclear intensities of the 18S* or 28S* RNA expressed from engineered plasmids, nuclei were segmented from the endogenous 28S FISH using cellpose with the nuclei model (inverted mask) and an expected object diameter of 300 px. The entire cell extents were then segmented from the endogenous 28S FISH stain (non-inverted mask) using the cyto2 cellpose model and an object diameter of 500 px. The whole-cell and nucleus objects were masked to ensure that each cell had a nucleus and vice versa. Then, the cell objects were masked with the nucleus to define the cytoplasm. The background subtracted endogenous 28S and engineered 18S* or 28S* RNA intensities were measured in the nuclei and cytoplasm.
Assumptions used for nucleolar phase-field modelling
As multiphase condensates, the organization of the nucleolar phases should be strongly impacted by the relative interfacial tensions (γi,j) between each of the phases (i,j), which is a measure of the energy per unit area associated with interfaces13,55,56,57,76. The relative interfacial tensions are expected to depend on the local concentrations of rRNA species and associated proteins (Fig. 5a), flux and biochemical nature of the underlying molecular species77.
Specifically, we modelled the affinities and localization of different species to be consistent with our experimental observations: nascently transcribed pre-rRNA in the FC/DFC boundary, 18S rRNA precursors before 5′ ETS cleavage in the DFC, and 18S rRNA and 28S rRNA precursors in the GC (Fig. 5a). Importantly, these rRNAs are not alone and form hundreds of interactions with proteins. Indeed, owing to its interactions with many assembly proteins and RNAs, the SSU processome represents a high-valency particle, of which the maturation, including the cleavage and degradation of 5′ ETS, releases 50 assembly factors to generate a pre-40S particle44,78,79,80.
A standard pairwise interaction term in the Flory–Huggins model encodes the affinity of rRNA to each nucleolar component and therefore the rRNA partitioning. Specifically, given our findings that U3-processing-deficient 18S precursors appear trapped within the DFC (Fig. 4b,d,h), our model assumes that these species have a high affinity for DFC, and a low affinity for the GC. Conversely, because 18S pre-rRNAs with normal processing flux into GC or nucleoplasm (Fig. 4b,d,h,i), we assume that 18S pre-rRNAs after 5′ ETS cleavage have a low affinity for the DFC and a higher affinity for the GC. Additional details are provided in Supplementary Note 2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.