
A 2022 regional study found that creatinine is an inadequate biomarker for kidney function in people in South Africa, Malawi and Uganda.Credit: Sandra Maytham-Bailey, ARK Study
In 2020, the Black Lives Matter movement forced the United States to reckon with systemic racism and its causes and manifestations. Driven partially by the disproportionate toll that the COVID-19 pandemic had on African Americans, the protests forced the medical community to question how to address racial health disparities, including how clinical practice itself can perpetuate systemic racism.
One prominent issue was the racial categories that are used in medical diagnostics. Although genetic ancestry can have ramifications for health, race is a social construct that does not map well to humanity’s genetic and physiological variability. “Utilizing that in the context of biologically driven decision-making is faulty. It’s fraught with pitfalls,” says physician Ebony Boulware, dean of Wake Forest University School of Medicine in Winston-Salem, North Carolina.
Medical tests that take race into account reinforce the idea that there are essential biological differences between ethnicities, which has long been debunked, Boulware adds.
Part of Nature Outlook: Medical diagnostics
Until 2020, US clinicians routinely interpreted diagnostic tests that estimate kidney function using equations that incorporated race — specifically whether a person was Black or not. In July 2020, the American Society of Nephrology and the US National Kidney Foundation announced a joint task force to review this situation1.
Kidney disease ranks among the starkest racial health disparities in the United States: kidney failure affects African Americans roughly four times more frequently than those of European ancestry.
The equations that underpin the medical tests under review used the level of creatinine in a person’s blood serum to estimate how quickly their kidneys filter blood — a metric known as estimated glomerular filtration rate (eGFR). Creatinine is a waste product that is made by the breakdown of skeletal muscle and was recognized as a potential marker of renal function in the 1920s and 1930s. Testing for it is cheap, quick and ubiquitous, essential characteristics of a diagnostic test. By contrast, getting a more accurate measured glomerular filtration rate requires injecting a person with an marker and then periodically collecting samples of blood or urine for several hours — which is expensive, time consuming and available only in specialist settings. eGFR equations are therefore central to detecting kidney disease (which is asymptomatic in its early stages), tracking disease progression and informing treatment decisions. An eGFR below 90 millilitres per minute signals kidney disease and a sub-15 eGFR indicates kidney failure. People are typically put on a waiting list for a kidney transplant when their eGFR reaches 20.
A coefficient for race was introduced into eGFR equations2 in 1999 — and included in a 2009 equation3 — to account for average differences in how creatinine levels relate to kidney function in Black people compared with other ethnic groups. Kidney function does not differ between groups, but African Americans tend to have higher creatinine levels than do white people and those from other ethnic groups. Creatinine levels are inversely related to eGFR, therefore introducing the coefficient meant that for a given creatinine value, Black individuals were assigned higher eGFR scores. That is, their kidneys were rated healthier than they would be for a white person with the same creatinine level.
At the time, this tweak to the formula was uncontroversial. “When they put this correction factor in, they were able to get closer to the true GFR,” says task force co-chair Neil Powe, a physician at the University of California, San Francisco. But then, Powe says, “it became socially unacceptable to use race in this biological measure”.
Studies also emerged showing that the coefficient was causing the healthiness of African Americans’ kidneys to be systematically overestimated4 (see ‘Kidney disease inequity’). “Having this racial designator in there,” Boulware says, “ironically and tragically put the vulnerable group at increased risk of not getting the recommended care, or getting it later.”
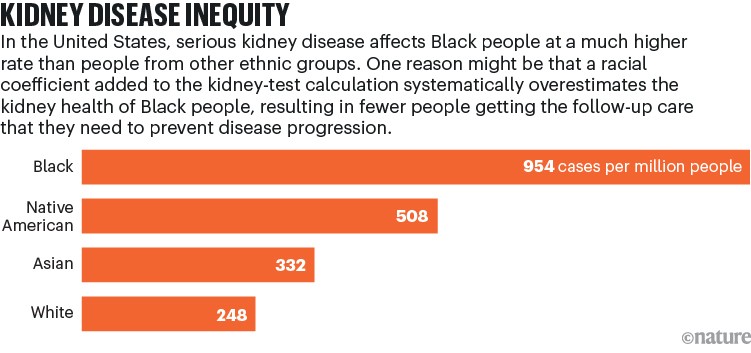
Source: NIH, NIDDK
The task force reviewed numerous ways to eliminate the race coefficient, issuing three recommendations1 in September 2021. First, when using creatinine-based tests, a new, race-free equation should be adopted. Second, when creatinine tests yield an uncertain result, physicians should also — if it’s available — use a second biomarker called cystatin C, which has never required a race coefficient. Third, research into the development of better kidney biomarkers should continue.
The first recommendation had an immediate effect. While the task force had been meeting, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) had independently developed a new, race-free creatinine eGFR equation, which was also published5 in September 2021. Following the task force’s recommendation, the United States quickly adopted this 2021 CKD-EPI equation. “I’ve never seen anything taken up so rapidly in medical care,” says Powe.
But adoption has been much patchier beyond the United States. European nephrologists have argued against using it. And in 2022, nephrologists in Africa questioned using creatinine as a biomarker altogether on that continent6.
A racialized biomarker
Biomarkers used to estimate GFR are molecules that steadily enter the blood from bodily tissues, which are then filtered out by the kidneys. The concentrations of such molecules in the blood reflect the equilibrium of these two processes.
To develop equations for using a biomarker on a large scale, researchers recruit volunteers, who should reflect the demographics of the population that will need the biomarkers. The level of the biomarker in a person’s blood is then plotted against each person’s measured GFR to derive an equation that best describes the relationship between the biomarker and kidney function. Acceptable performance for a creatinine equation typically gives an eGFR within 30% of a person’s measured GFR in 80–90% of cases5.
Researchers can also check whether splitting their sample by definable characteristics — such as sex, age, weight or race — yields modified equations that better predict measured GFR for each subgroup.
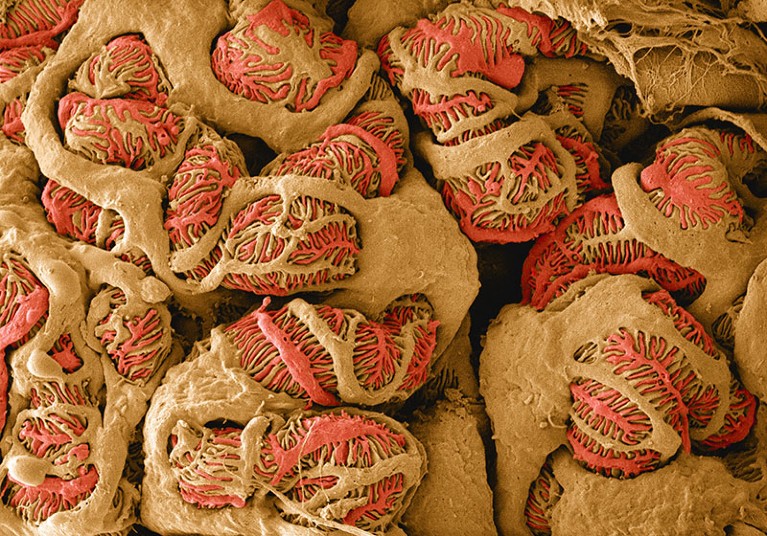
The glomerulus, a network of small blood vessels in the kidney, plays an important part in filtering blood. Commonly used methods to measure glomerular filtration rate — a metric of how well the kidneys function — have led to systematic overestimation of kidney health in Black people, sometimes leading to delayed treatment.Credit:Thomas Deerinck NCMIR/SPL
Because creatinine comes from the breakdown of skeletal muscle, factors that influence how muscular a person is — such as their sex and age — were always prominent concerns when it was developed as a biomarker. The first globally used equation — the 1976 Cockcroft–Gault equation, based on data from just 249 adult white men7 — included coefficients for age, weight and sex (the latter based on extrapolation from other work).
But in the 1990s, large US population studies8 showed that African Americans typically have higher creatinine levels than do other Americans. “It’s been replicated in every study that has measured GFR carefully,” Powe says. A desire to account for this led to the introduction of the race coefficient to the eGFR equation2 in 1999. The coefficient is called the MDRD, after the Modification of Diet in Renal Disease study that it emerged from. The MDRD race coefficient was 1.21, meaning that a Black person’s eGFR (and hence kidney function) was estimated to be 21% higher than that of a person from another ethnic group with the same creatinine level. Ten years later, after larger studies, the 2009 CKD-EPI equation was introduced3; this largely superseded the MDRD equation, and lowered the racial coefficient to 1.16.
Some researchers say too little effort has been made to understand the biology of creatinine and its differing levels between US people of African and European backgrounds. “The ball was dropped trying to figure out why there’s that difference,” says task-force member Marva Moxey-Mims, a paediatric nephrologist at the Children’s National Hospital in Washington DC. “People started making up explanations.”
Researchers and physicians often assumed that Black Americans simply had more muscle mass than those in other groups, but the few studies9 that tested this notion did not support it. Powe says that diet and lifestyle play a part, adding: “We still don’t know to this date why creatinine levels are higher.”
The MDRD and 2009 CKD-EPI equations were adopted worldwide, albeit with certain caveats. In Japan, for example, local validation studies introduced a coefficient of roughly 0.8 for people of Japanese descent10.
And, when several European countries imported these US equations, they did so without the race coefficient (the UK being a notable exception). “We did not believe in the race conversion factor because it suggests that Black people are different,” says Ron Gansevoort, a nephrologist at the University of Groningen in the Netherlands. Besides, the eugenic atrocities of the Second World War led nearly all European countries to adopt policies that removed any indication of religion or ethnicity from people’s medical records. “We could not use it,” says Gansevoort, “even if we wanted it.”
Deracializing a biomarker
Around 2016, pressure groups led by US medical students started calling for eGFR equations to be deracialized. Rejecting race as a valid medical category, they proposed to simply eliminate the coefficient from existing equations. By 2020, several US hospitals had done just that, followed by the United Kingdom in 2021.
But Powe and the task force argued that dropping the coefficient risked systematically underestimating Black people’s kidney function, leading to over-diagnosis of kidney disease -and preventing Black individuals from accessing a range of important drugs that require healthy kidneys. Also, Powe says, “by dropping the coefficient, you’re actually dropping all the creatinine values for African Americans and you’re ignoring them. If you do that in all of medicine, you just say, ‘Let’s normalize to the white race’.”
Detecting hidden brain injuries
The task force instead backed CKD-EPI’s revised 2021 equation, which pooled data from Black and non-Black people to derive a single equation that best fitted both groups5. Developing that meant weighting the balance of volunteers to account for the racial disparities in kidney disease. Accordingly, 31.5% of the people in the data set used to develop the 2021 CKD-EPI equation were Black, much higher than the 14% in the US population. But Powe says this higher percentage approximates how many Black people in the United States have kidney failure. “If you’re going to be using this for health equity,” he says, “you should overpopulate it with African Americans.”
This decision has a major consequence. “Based on that mix,” Powe says, “what the equation does is change the estimated GFR not only for African Americans. It changes it for everyone.” For Black individuals, eGFR goes down fractionally. For other people, it rises slightly.
Simulations of the 2021 CKD-EPI equation’s effects predict that more than 400,000 Black Americans would gain a new diagnosis of kidney disease. Ideally, this would lead to better care. However, the equation could also lead to more than five million Americans from other ethnic groups no longer being diagnosed with the disease11.
The United States versus the world
The need to shift the eGFR of everyone who needs a creatinine test has made other countries and regions hesitant about using the 2021 CKD-EPI equation. A committee of nephrologists in Canada voted to implement it. But European groups‚ serving a continental population in which Black people make up only around 2%, decided against. Most European nephrologists are sticking with the 2009 CKD-EPI, without the race modifier.
Some Europeans are critical of the deracialized 2021 equation. “Because you kick out one variable that explains variance — the race variable — it becomes worse for both white and Black people,” says Hans Pottel, a nephrologist at the Catholic University of Leuven (KU Leuven) in Kortrijk, Belgium. “This is a mathematical construction. It has nothing to do with racism.”
In the United Kingdom, an ongoing study that includes various ethnic groups aims to determine which available equation would best serve the country’s population12.
One of those is the European Kidney Function Consortium (EKFC) equation that Pottel has been helping to develop since 2014. It uses the average creatinine levels of various demographic populations — defined by sex, age, geographical location and ethnicity — as a reference, appropriately normalizing tested individuals’ creatinine scores before calculating their eGFR13. The overall equation would be globally applicable but could be adapted to local populations.
This, Pottel says, is not the same as using race as a category. “It has nothing to do with the colour of the skin. It has to do with different populations.” For example, his team has found that creatinine levels vary considerably between populations that would be classified as Black in Europe and the United States compared with those in the Democratic Republic of the Congo and Côte d’Ivoire14.
Elsewhere in Africa, research raises even deeper concerns about kidney function testing. The largest regional study15 of its kind, published in 2022, looked at more than 3,000 people from Malawi, Uganda and South Africa, and found that no available creatinine-based eGFR equation indicated people’s kidney health accurately. Aligning with previous smaller studies, all equations that are currently in use systematically overestimated kidney function in these three countries — and that using the 2009 CKD-EPI equation with its the race coefficient made the overestimation worse. “Creatinine is a lousy biomarker in our setting,” says study leader June Fabian, a nephrologist at the University of the Witwatersrand in Johannesburg, South Africa.
A radiographer performs an investigative kidney ultrasound as part of a regional study in South Africa, Malawi and Uganda.Credit: Sandra Maytham-Bailey, ARK Study
Fabian’s co-author, Robert Kalyesubula, a nephrologist at Makerere University in Kampala, explains that many African populations differ profoundly from those in which creatinine has been validated as a kidney function marker. For example, people from Malawi, Uganda and South Africa typically have less muscle mass, eat less protein and are more often malnourished than are African American populations (who largely have West African ancestry) for whom creatinine levels have been much better characterized. Moreover, the kidney diseases affecting low-income African countries also differ from the late-life, hypertension- and diabetes-related kidney diseases that are dominant in high-income countries. These factors all contribute to creatinine’s inadequacy as a biomarker in the countries that Kalyesubula and Fabian studied.
Because existing equations overestimate kidney function, Fabian says, African countries’ previous adoption of them – mainly the 2009 CKD-EPI equation without the race coefficient — has probably cost lives. “We’ve used them for decades,” she says. “The consequence of that is that we’re missing a lot of people who have renal disease.”
“The American lens in nephrology dominates all the research, a lot of the funding and the narrative,” says Fabian. “In an American nephrologist’s head, an African American represents the rest of Africa. That’s one of the huge biases we’ve been trying to challenge. The genetic diversity in Africa is phenomenal.”
The cystatin alternative
Fabian and Kalyesubula did, however, find that in these three African nations, the alternative biomarker cystatin C provided a better way to measure eGFR15.
Cystatin C is a metabolite that is released into the blood by every cell in the body and filtered out by the kidneys. It has been a recommended diagnostic tool since the early 2000s. With no systematic differences in cystatin C levels being evident between ethnic groups, race modifiers have never been needed for it to provide useful results.
The main barriers to shifting to its more widespread use are cost and availability. Fewer places currently offer cystatin C testing and it is at least three times more expensive to measure than is creatinine. In the United States, this is a matter of roughly US$18 versus $5, which for 250 million annual tests would amount to more than $3 billion in extra health-care costs. In some countries, testing for cystatin C can be ten times as costly as for creatinine.
Although there are technical reasons why cystatin C testing might never be as cheap as creatinine testing, costs might reduce. “Like anything else,” Moxey-Mims says, “the more you use it, the more the price will come down.”
Cystatin C is not unequivocally a better marker overall, however. Gansevoort notes that smoking severely distorts cystatin C results. Obesity, inflammation and thyroid disorders can also have an effect. “Every marker has its advantages and disadvantages,” he says. Generally speaking, clinicians should be mindful that eGFR gives only an estimate of kidney function, Gansevoort notes.
The CKD-EPI group and other researchers have shown that combining creatinine and cystatin C data provides the most accurate estimation of GFR in many contexts5. However, Fabian and Kalyesubula found cystatin C alone to be most accurate.
Potentially, even better blood-borne markers exist — and, as the joint task force recommended, research into this possibility should continue. One option under investigation is to use inexpensive urine tests that can detect kidney damage. But all these techniques still need extensive research and investment to bring them to maturity and enable widespread use.
The measure of a measure
For now, a global consensus on kidney testing remains elusive. In April this year, new guidelines16 issued by the nephrology organization Kidney Disease: Improving Global Outcomes (KDIGO) stated that “use of race in the computation of eGFR should be avoided”.
But KDIGO did not advocate a single preferred equation. Instead, it recommended only that a validated eGFR equation be used, and that this choice should be uniform across geographical regions.
Consequently, there is an apparent impasse. As individual regions confront their unique cultural, historical, financial, demographical and infrastructural challenges, national and regional differences in kidney testing might persist for some time.
For the United States in particular, one key question is whether changing eGFR equation protocols will lead to greater equity in health care. One prominent outcome is the work that is addressing the longer waiting times for kidney transplants that Black Americans have historically experienced.
Because race coefficients inflated African Americans’ eGFR scores, when their kidneys were failing, they reached the threshold for a transplant later than they would have done if the race coefficient had not been used to assess their kidney function. And this probably delayed transplants for many African Americans. In 2022, the Organ Procurement and Transplantation Network (OPTN), the public–private organization that oversees transplant policy in the United States, recommended that African Americans’ eGFR be recalculated without race coefficients, says Martha Pavlakis, a nephrologist at Beth Israel Deaconess Medical Center in Boston, Massachusetts, and vice-chair of the OPTN’s kidney-transplant committee. Then, in 2023, the OPTN acted to adjust the priority of African Americans currently awaiting transplants, whose previously coefficient-inflated eGFR scores had lowered their priority and extended their wait times. Retroactively lowering these eGFR values has cut the transplant waiting times for affected people by an average of two years17, Pavlakis says.
The race-free 2021 CKD-EPI equation no longer requires this adjustment. Yet, currently, there are surprisingly few data with which to assess its affect.
Sherri Rose, who studies medical algorithms at Stanford University in California, explains that diagnostic equations occupy a curious place in medicine. Unlike treatments and the biochemical tests underpinning diagnostics, equations themselves don’t require regulatory approval. This means trials of an equation to determine its effect on clinical outcomes are not required before its clinical use. Rose led the only study18 so far on the effects of the 2021 CKD-EPI equation. It examined the health records of more than 500,000 people whose kidney function had been tested in a health-care system, Stanford Health Care, for three years before and two years after the equation was introduced. The main question was whether the new equation had benefited Black people by getting them more referrals to see nephrologists.
The answer was that it had not.
That is not a definitive finding, of course. Rose says the result concerns just one health-care system, which might not represent US health care in general. She urges other centres to run studies and to look at other health outcomes, and cautions that it might take five to ten years to see the equations having an effect on outcomes.
But for now, her study raises a cautionary flag about whether the change in formula alone is sufficient to achieve greater health equity. “A change of formula is a big headline,” she says. “It’s a quick ‘win’. But it doesn’t necessarily solve the problem.”




