Mice
All investigations concerning mouse work had local approval and all procedures conformed to the guidelines from Directive 2010/63/EU of the European Parliament on protecting animals used for scientific purposes. In detail, all animal experiments were conducted according to the German law of animal protection and in agreement with the approval of the local institutional animal care committee (Landesamt für Natur, Umwelt und Verbraucherschutz (LANUV), North Rhine-Westphalia, Az 81-02.04.2019.A146 and Az 2024-A314). Mice were housed under specific pathogen-free conditions with 12-h light–dark cycle, at 21 °C, 55% relative humidity, and with food and water provided ad libitum. Animals were euthanized using liver or heart perfusion after anaesthesia injection. C57BL/6JRcc was used as the WT strain. To generate the double fate mapper Tnfrsf11aCre (ref. 56); Rosa26LSL-YFP (JAX stock #006148)57; Ms4a3FlpO;Rosa26FSF-tdT (JAX stock #032864)58, we bred Tnfrsf11aCre/+;Rosa26FSF-tdT/FSF-tdT animals with Ms4a3FlpO;Rosa26LSL-YFP/LSL-YFP animals (see Extended Data Fig. 4a for description of the Ms4a3FlpO locus). KC-DTR (Clec4fDTR/+) mice have been previously described32. All mice were originally of the C57BL/6J background and crossed to C57BL/6Jrcc for 2–5 generations. To generate Hif1aflox/flox;LysMCre/+, the Hif1aflox/flox (JAX stock #007561)59 and LysMCre/+ (JAX stock #004781)37 were used (both kept on the C57BL/6J background after import). All lines were back-crossed to their respective WT line (C57BL/6J for LysMCre/+;Hif1aflox/flox and C57BL/6Jrcc for all other lines) once per year. Mice were genotyped according to protocols provided by JAX or donating researchers. For all experiments, male mice were analysed with the exception of the double fate mapper, where both male and female mice were used, and ex vivo-isolated hepatocytes, where only young female mice were used to assure a lean and metabolically healthy state.
Mouse maternal obesity model
For WT cohorts, 3–4-week-old C57BL/6JRcc female mice were put on a CD (sniff, E15748-047) for 2 weeks. Subsequently, female mice were separated into two groups and received either a HFD (sniff, E15742-347) or a CD for 8 weeks. For metabolic assessment, the HOMA-IR was used60. Glucose was measured using GlucoCheck GOLD with stripes (11864873 and 11864933), and insulin was measured via ELISA (EMINS, Thermo Fisher). Only mice with significantly higher HOMA-IR were used to generate offspring. Females were mated with males fed with a CD overnight. The day of vaginal plug formation was estimated as embryonic developmental day 0.5 (E0.5) post-coitum. Females were always placed back in their cages with a HFD after overnight mating. All offspring were cross-fostered to females on the respective diet. The litter size was standardized to a maximum of six pups per foster mother on the day of birth. In addition, whenever possible, litters from different biological mothers were cross-fostered to the same foster mother to ensure consistency and minimize maternal variability. Cross-fostering took place also between mothers on the same diet to exclude stress-mediated effects of cross-fostering. After 3 weeks of lactation at postnatal day 21 (P21), offspring were weaned into their post-weaning cage (CD or HFD). Here offspring from maternal lean and maternal obese groups were mixed to minimize effects caused by cage-specific microbiome differences. In addition, during the post-weaning diet, bedding stemming from cages with the same diet was mixed weekly between cages (typically 10–20 cages at any time) to further reduce cage-specific effects related to the microbiome.
Mice were euthanized and used in the experiment at weeks 11–13, unless otherwise stated.
Adoptive transfer of KCs for KC-DTR mice
Maternal obese KC-DTR male offspring were injected at P0 with 50 ng diphtheria toxin and injected at P1 through the temporal vein under ice-induced anaesthesia with 106 bone marrow monocytes and HSPCs isolated from Rosa26mTmG mice. Bone marrow monocytes and HSPCs were isolated as following: Rosa26mTmG mice were killed through cervical dislocation, and their tibias and femurs from the hindlimbs were freed. Bone marrow was flushed out with DMEM (Pan Biotech) and filtered through a 70-μm strainer on ice. Cells were then blocked with FACS buffer (0.5% BSA and 2 mM EDTA in PBS) containing 2% rat serum and stained with biotinylated antibodies (anti-Ly6G, CD19, CD3, Ter119, Nkp45 and F4/80) for 30 min at 4 °C. Monocytes and HSPCs were then negatively selected using MojoSort Streptavidin Nanobeads (BioLegend) following the manufacturer’s manual.
Preparation of cell suspension for flow cytometry and cell sorting
Adult mice were anaesthetized and perfused with ice-cold PBS. P0 mice were killed by decapitation. For flow cytometry analysis of hepatic myeloid cells, 200–300 mg of adult or half of a P0 liver was collected, cut into small pieces and incubated in a digestion mix (PBS containing 1 mg ml−1 collagenase D (11088858001, Roche), 100 U ml−1 DNase I (DN25, Sigma-Aldrich), 2.4 mg ml−1 of dispase (17105041, Gibco) and 3% fetal calf serum (FCS; Invitrogen) for 30 min at 37 °C before mechanical disruption through a 100-μm filter. The cell suspension was diluted in 3 ml of FACS buffer (0.5% BSA and 2 mM EDTA in PBS) and centrifuged at 50g for 3 min to remove hepatocytes. Then, the supernatant containing myeloid cells was collected and centrifuged at 370g for 7 min at 4 °C. For bone marrow, one leg was isolated, and both the tibia and the femur were cleaned from surrounding tissues and cut at the ends. The bones were flushed with 5 ml of ice-cold FACS buffer. Cells were centrifuged at 370g for 7 min at 4 °C. Pellet was resuspended in red blood cell lysis buffer (155 mM NH4Cl, 12 mM NaHCO3 and 0.1 mM EDTA) and incubated for 3 min on ice. Then, 5 ml FACS buffer was added, cells were resuspended and centrifuged at 370g for 7 min at 4 °C. Cell pellet was resuspended in FACS buffer containing purified anti-CD16/32 and 2% rat serum (liver) or 2% rat serum only (bone marrow) and incubated for 15 min at 4 °C. Samples were immune-stained with antibody mixes for 30 min at 4 °C. The complete list of antibodies used can be found in Supplementary Table 8. Data were acquired with FACSymphony A5 (BD Biosciences) or LSRII and analysed in FlowJo Software. For sorting of KCs and hepatocytes, liver cell suspension was prepared as described above with the following modifications: first, 1 ml twice-concentrated digestion mix was used for tissue digestion; second, digestion was performed at room temperature and centrifugation at 50g for 3 min was omitted to retain hepatocytes; and last, all steps were performed with buffers containing 1 mM of flavopiridol (F3055, Sigma-Aldrich). Cells were stained by antibody mixes and sorted using FACS ARIA III cell sorter (BD Biosciences) directly into 500 μl lysis buffer (79306, Qiagen). For KC metabolic flow cytometry assays, single-cell suspensions were blocked with anti-CD16/32 (1%) and rat serum (2%) in FACS buffer, followed by surface marker staining for 30 min at 4 °C. Cells were washed and fixed in 1% paraformaldehyde for 5 min and then permeabilized for 15 min with PBS supplemented with 0.4% Triton X-100 (X100, Sigma-Aldrich). Intracellular staining was performed for 1 h at 4 °C. Intracellular antibodies were conjugated in-house using lightning-link conjugation kits (Abcam) as previously described26. Cells were analysed on an ID7000 7-laser Spectral Cell Analyzer (Sony Cooperation).
Histology
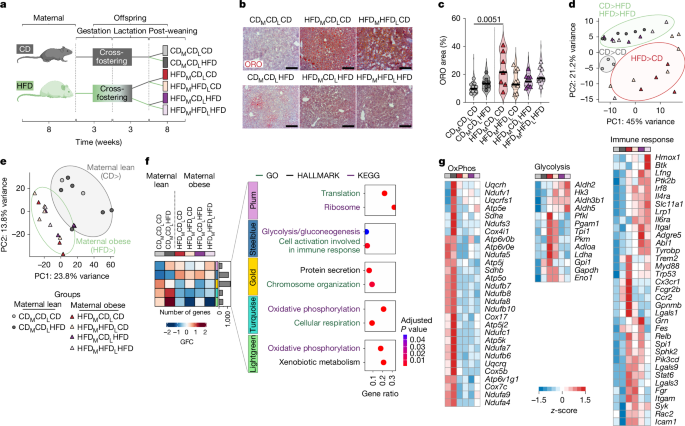
For neutral lipid staining, cryo-preserved liver blocks were cut at 10 µm thickness and dried for 1 h at room temperature. Lipid staining was performed using an Oil-red-O Stain Kit (O0625, Sigma-Aldrich) according to the manufacturer’s instructions. The sections were then stained with haematoxylin for 5–10 min to visualize nuclei, rinsed with distilled water and mounted with Kaiser’s glycerol gelatine (pre-heated at 55 °C; 6474.1, Carl Roth). Images were taken with an Axio Lab.A1 microscope (Zeiss). For ORO staining quantification, 2–10 images of each sample with a ×40 objective were taken and quantification was performed using QuPath software (v0.5.1). For haematoxylin and eosin (H&E) staining, paraffin-embedded liver blocks were cut at 5 µm thickness and heated for 1 h at 65 °C before staining. Sections were deparaffinized by two steps of xylol and descending series of alcohol and rinsed in distilled water. Next, sections were stained with haematoxylin solution for 1.5 min and neutralized by running tap water, followed by alcoholic eosin staining for 2 min. Sections were then dehydrated by an ascending series of alcohol and two steps of xylol and mounted with Entellan (1.07961.0100, Millipore). Images were taken with the Axio Lab.A1 microscope. Quantification of droplet adiposity in liver cells in H&E-stained histological sections was performed manually using a light microscope without the support of digital imaging or software tools. From each section, 10 random high-power fields were selected at ×10 magnification to ensure a representative selection of liver parenchyma while avoiding areas of artefacts or tissue damage. Hepatocytes were analysed for the presence of lipid droplets. All hepatocytes in each selected field were counted and classified as either normal (without lipid droplets) or lipid laden (with lipid droplets recognizable as intracellular vacuoles). The percentage of lipid-laden hepatocytes was calculated for each field by dividing the number of hepatocytes with lipid droplets by the total number of hepatocytes in the field and multiplying the result by 100. The percentages of all analysed fields were averaged to obtain a representative value for each sample. For immunofluorescence staining, cryo-preserved liver blocks were cut at 10 µm thickness, dried for 1 h at room temperature and permeabilized with PBS-T (0.4% Triton X-100; X100, Sigma-Aldrich) for 1 h at room temperature. Thereafter, tissue was blocked with PBS-T containing 2% BSA (37525, Thermo Scientific) and 2% donkey serum (D9663, Sigma-Aldrich) for 2 h at room temperature, followed by primary antibody staining overnight at 4 °C, and secondary antibodies hosted in donkey for 2 h at room temperature. Nuclei were stained with DAPI (42281, BioLegend). Immunofluorescence images were acquired with a Zeiss LSM Airyscan 880 microscope (Zeiss) and processed with Fiji61.
Direct infusion lipidomics
Livers were isolated from ice-cold PBS-perfused mice and snap-frozen in liquid nitrogen. Ten milligrams were homogenized in ddH2O using a Precellys homogenizer (Peqlab Biotechnology). For lipid extraction, 50 µl of the homogenate was added to 500 µl extraction mix (CHCl3:MeOH 1:5 containing the following internal standards: 210 pmol phosphatidylethanolamine (PE)(31:1), 396 pmol phosphatidylcholine (PC)(31:1), 98 pmol phosphatidylserine(31:1), 84 pmol phosphatidylinositol(34:0), 56 pmol phosphatidic acid(31:1), 51 pmol phosphatidylglycerol(28:0), 28 pmol cardiolipin(56:0), 39 pmol lysophosphatidic acid(17:0), 35 pmol lysophosphatidylcholine (LPC)(17:1), 38 pmol lysophosphatidylethanolamine(17:0), 32 pmol ceramide(17:0)), 99 pmol sphingomyelin(17:0), 55 pmol glucosylceramide(12:0), 14 pmol monosialodihexosylganglioside(18:0-D3), 339 pmol triacylglycerol(50:1-d4), 111 pmol cholesteryl ester(17:1), 64 pmol diacylglycerol(31:1), 103 pmol monoacylglycerol(17:1), 724 pmol cholesterol(D6) and 45 pmol carnitine(15:0)) and subsequently sonicated for 2 min and centrifugated at 20,000g for 2 min. The supernatant was collected into a new tube, and 200 µl chloroform and 800 µl 1% AcOH water were added. The sample was shaken and centrifuged for 2 min at 20,000g. The upper aqueous phase was discarded and the lower phase was transferred into a new tube and evaporated in a speed vacuum concentrator (45 °C at 10 min). Spray buffer (500 µl of 8:5:1 2-propanol:MeOH:H2O and 10 mM ammonium acetate) was added to the sample and sonicated for 5 min, infused at 10 µl min−1 into a Thermo Q Exactive Plus spectrometer equipped with the heated electrospray ionization II ion source for direct infusion lipidomics. MS1 spectra (resolution of 280,000) were recorded in 100-m/z windows from 250 to 1,200 m/z (positive) and 200 to 1,700 m/z (negative) followed by recording tandem mass spectrometry spectra (resolution of 70,000) by data-independent acquisition in 1-m/z windows from 200 to 1,200 (positive) and 200 to 1,700 (negative) m/z. Raw files were converted to .mzml files and imported into and analysed by LipidXplorer software (v1.2.8.1)62 using custom mfql files to identify sample lipids and internal standards.
Lipidomics analysis
Raw measurements of the lipidome were quantified in relation to internal standards measured simultaneously on the same instrument. The resulting values were normalized by the amount of tissue to obtain measurements in pmol mg−1. All lipid species that remained constant across all samples were removed from the dataset. In addition, species belonging to the triacylglycerol class with odd numbers of double bonds were excluded, as they were unlikely to originate from mice. This resulted in a set of 19 lipid classes: cholesteryl ester, ceramide, diacylglycerol, dihexosylceramide, hexosylceramide, LPC, ether-linked LPC, lysophosphatidylethanolamine, monoacylglycerol, phosphatidic acid, PC, ether-linked PC, PE, ether-linked PE, phosphatidylglycerol, phosphatidylserine, sphingomyelin, unsaturated fatty acyl tails of triacylglycerol (unsat) and saturated fatty acyl tails of triacylglycerol (sat). Following the selection process, the dataset consisted of 476 metabolites for C57BL/6Jrcc mice (69 dropped) and 443 metabolites for the HIF1α model (31 dropped). Within each sample, the species belonging to the same class were aggregated by calculating the mean. The results were then summarized by computing the mean per condition. Log2 fold changes were calculated by comparing each condition to the control condition CDMCDLCD. For each lipid class, the conditions were assessed for significant deviations from the control condition using the lm function in R. Subsequently, a hierarchical clustering analysis was performed on the resulting dataset, both row wise and column wise, based on the Euclidean distance with complete linkage. The colour legend for the HIF1α mouse model is not evenly spaced around zero. This deviation arose from significant changes with low-effect sizes. To still indicate the direction of change, the space around zero was adjusted accordingly. The colour histogram shown represents the entire set of lipid species, not just the selected species shown in the main figure. The value of 1 was added to the data before performing the logarithmic transformation (log2) and performing PCA.
Multiplexed cytokine and chemokine assay
The Immune Monitoring 48-Plex Mouse ProcartaPlex Panel (EPX480-20834-901, Invitrogen) was used. Of sera, 25 µl was thawed and analytes were evaluated using Luminex xMAP system. All reagents were prepared and used according to the manufacturer’s instructions. Cytokines that were below the detection threshold in more than 80% of the samples were not plotted.
The values displayed within the heatmap are log10 + 1 transformed and ordered by decreasing overall mean.
Library preparation and RNA-seq
cDNA library for sequencing was prepared as described in the SMART-Seq2 protocol63. The mRNA was extracted and primed utilizing poly-T oligonucleotides and converted into cDNA by SMART reverse transcription. Pre-amplification was performed by SMART ISPCR, followed by fragmentation using the Nextera XT DNA Library Preparation kit (Illumina), amplification and indexing. Library fragments were then selected by size (300–400 bp) and purified using SPRIBeads (Beckman-Coulter). The Agilent high-sensitivity D5000 assay was used to measure the size distribution of cDNA libraries on a Tapestation 4200 system (Agilent). Quantifying cDNA libraries was performed using a Qubit high-sensitivity dsDNA assay (Thermo Fisher). Sequencing was performed using a 75-bp single-end setup on the NextSeq500 system (Illumina), applying NextSeq 500/550 High Output Kit (v2.5; Illumina). Library fragments were then selected by size (300–400 bp) and purified using SPRIBeads (Beckman-Coulter). The Agilent high-sensitivity D5000 assay was used to measure the size distribution of cDNA libraries on a Tapestation 4200 system (Agilent). The cDNA libraries were quantified using a high-sensitivity dsDNA assay (Qubit). To quantify the abundances of transcripts from the bulk RNA-seq data, Kallisto pseudo alignment was applied64.
Transcriptomics analysis
The raw transcriptome files were prepared for Kallisto import to DESeq2 using the genecode annotation M16 (https://www.gencodegenes.org/mouse/release_M16.html) to correct for library size based on the provided average transcript length. Outliers (eight from the C57BL/6Jrcc dataset and three from the Hif1a dataset) were identified on the basis quality assessment and filtering of low-quality samples. The set of genes was further refined to include only protein-coding genes that were non-constant across all present samples. Low-expressed genes, defined as those with less than 10 counts in 25% of the samples, were also removed. PCA was performed on the variance-stabilized counts. DESeq2 (ref. 65) analysis was conducted to identify DEGs within each cell type. The default settings in DESeq2 were used. A gene was called differentially expressed if its corresponding Benjamini–Hochberg adjusted P value was less than 0.1 for the tested contrast. For the KC P0 DEGs subjected to transcription factor inference analysis, the absolute LFC threshold was set to 2. The DEGs were separated into two sets based on the sign of the LFC. Each set of DEGs was subjected to an over-representation analysis to identify enriched GO terms, HALLMARK pathways and KEGG pathways using the respective functions in clusterProfiler66. To reduce observed redundancy in the GO terms, the ‘simplify’ function in clusterProfiler was used, which calculates the similarity among enriched terms based on their information content and selects a representative term with the lowest adjusted P value67. The universe for the over-representation analysis consisted of all genes used for the differential expression analysis. For the ligand–receptor analysis, DEGs with an absolute LFC greater than 2 in the KC set were subsetted to include ligands recorded in the CellTalk database68. For the hepatocyte dataset, all expressed genes (more than 10 counts in Hif1a-WT or Hif1a-KO HFDMCD conditions; not necessarily differentially expressed) were subsetted to include recorded receptors within the same database. Interactions between differentially expressed ligands from KCs and expressed receptors from hepatocytes were extracted from the CellTalk database and plotted. The order of ligands was based on their LFC from lowest to highest. A co-expression network analysis using hCoCena25 was conducted on the filtered and DESeq2 normalized counts, following the provided showcase Notebook25. hCoCena calculates pairwise correlations among the top most varying genes (the number needs to be user-specified) and constructs a network where vertices represent genes with at least one edge characterized by a correlation above a specified threshold. Here hCoCena was performed using the top 5,000 genes as input and all edges were kept with a correlation above 0.7. The resulting network, with 4,214 nodes and 286,853 edges, was then clustered using the Leiden algorithm69. Clustering was performed with 100 iterations. Each node that was advised to more than three clusters during the process was disregarded in the proceeding cluster analysis. The identified gene sets were summarized using Group Fold Change25 for each condition and displayed on a heatmap. Each cluster set was subjected to an over-representation analysis, with the hCoCena-input genes specified as the universe for testing. Manually selected terms from GO, KEGG and HALLMARK sets are displayed for each cluster to avoid term cluttering. For selected enriched terms, their intersection with the cluster genes was recovered, namely, ‘oxidative phosphorylation’ with turquoise and lightgreen, ‘glycolysis’ and ‘cell activation involved in immune response’ with steelblue. The resulting set of genes was further manually reduced. The mean expression values for those intersectional genes were displayed and scaled across each condition. The columns were hierarchically clustered on the basis of the Euclidean distance with complete linkage. Transcription factor inference analysis was conducted using the CollecTRI38 framework, which integrates information from multiple sources, including RegNetwork70, ChEA3 (ref. 71), Pathway Commons72 and DoRothEA73. Within this analysis, a linear model was used to estimate the transcription factor activity. The model predicts the value of the DEGs solely based on the interaction weights between transcription factors and their respective target genes derived from CollecTRI. The final score was the t-value of the slope of the fitted model. A positive value indicates an active transcription factor. This analysis was conducted using decoupler’s74 functionality.
snRNA–ATAC-seq
Nuclei were isolated from approximately 50 mg of snap-frozen adult liver tissue to enable simultaneous snRNA-seq and snATAC-seq, capturing both transcriptional profiles and chromatin accessibility. Nuclei were prepared using the Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Kit (1000283, 10X Genomics). In detail, snap-frozen liver tissue was first cut on dry-ice into small pieces and homogenized with a pestle in homogenization buffer containing 320 mM sucrose, 5 mM CaCl2, 3 mM Mg-acetate, 10 mM Tris, 0.1 mM EDTA, 0.1% IGEPAL CA-630 (Sigma), 0.1 mM phenylmethylsulfonyl fluoride (Sigma), 0.2 U μl−1 RNase inhibitor (New England Biolabs), 1 mM flavopiridol and 1 mM β-mercaptoethanol (ITW Reagents) in nuclease-free water. Homogenate was then passed through a 70-μM cell strainer and mixed with equal volume of gradient medium containing 50% OptiPrep (StemCell Technologies), 5 mM CaCl2, 3 mM Mg-acetate, 10 mM Tris-HCl (pH 8), 0.1 mM phenylmethylsulfonyl fluoride (Sigma) and 1 mM β-mercaptoethanol (ITW Reagents) in nuclease-free water. Diluted homogenate was slowly laid atop a cushion (75% of the volume of diluted homogenate) containing 29% OptiPrep, 77.5 mM KCl, 15.5 mM MgCl2, 31 mM Tris-HCl (pH 8) and 129.2 mM sucrose in UltraPure H2O and centrifuged with low acceleration and maximum deceleration at 10,000g at 4 °C for 30 min. The pellet containing nuclei was resuspended in 1X PBS containing 1% BSA, 0.2 U μl−1 RNase inhibitor (New England Biolabs) and 1 mM flavopiridol, stained with 7-AAD and purified by fluorescence-activated cell sorting using FACS ARIA III (BD Biosciences) for high-quality, monoploid and 7-AAD nuclei. For nuclei permeabilization, approximately 500,000 sorted nuclei were incubated for 2 min in a 0.1× lysis buffer (10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 0.1% IGEPAL CA-630, 0.1% Tween-20, 0.01% digitonin, 1 mM dithiothreitol, 1% BSA and 1 U μl−1 RNase inhibitor, with 1 μM flavopiridol in nuclease-free water). Following permeabilization and washing steps, nuclei concentration was adjusted to 5,000 nuclei per microlitre in ice-cold 1× nuclei buffer (2000153/2000207, 10X Genomics) and were incubated in a transposition mix containing a transposase, allowing adapter sequences to be added to the ends of DNA fragments. Furthermore, 16,000 nuclei were used for analysis with the Next GEM Single Cell Multiome ATAC + Gene Expression Kit (PN-1000230, 10X Genomics) and Gel Beads in Emulsion (GEMs) using the Chromium Next GEM Chip J Single Cell Kit. After GEM reverse transcription (GEM-RT) cleanup, pre-amplification of the sample was performed, producing material for both ATAC library construction and cDNA amplification for gene expression library construction. During the reverse transcription reaction, cDNA tagging was achieved with 16-nucleotide barcodes and 10-nucleotide molecular identifiers. The pre-amplified product was split into two aliquots: 40 μl for ATAC library construction and 35 μl for further cDNA amplification. For snRNA-seq library preparation, 25% of the total cDNA was used to generate gene expression (GEX) libraries. Libraries were prepared and sequenced according to the manufacturer’s protocols: snATAC libraries used the 10X Genomics Single Index N Set, and snRNA-seq libraries used the Dual Index TT Set A. Pooled and barcoded libraries were sequenced on an Illumina NextSeq 2000 system with P3 flow cells, achieving an average depth of 30,000 reads per nucleus for both snRNA-seq and snATAC-seq libraries. The sequencing quality was assessed using FastQC (v0.12.1) executed within a containerized environment provided by biocontainers using Podman. All files passed initial quality assessment. Following assessment, Cell Ranger ARC (v2.0.0; 10X Genomics) was used for simultaneous alignment and quantification of both modalities. The cellranger-arc count pipeline was run according to the manufacturer’s instructions using the 10X Genomics-provided reference dataset refdata-cellranger-arc-mm10-2020-A-2.0.0, which is based on the mm10 genome assembly and including introns. The automatically obtained quality assessment alerted for both conditions that the amount of valid ATAC barcodes was at 54% (ideally more than 80%). Despite the low proportion, both datasets were used because other critical ATAC quality metrics, such as fragment count and enrichment scores, met acceptable thresholds. Single-cell RNA-seq and ATAC-seq data were processed using mainly the Seurat and Signac packages in R. Both modalities were extracted from the 10X Genomics ‘filtered feature matrix file’, in which count data were used for additional filtering of low-quality cells, before using MACS3 to perform the peak calling within the remaining cells to reduce computational time. Applied thresholds were: cells with RNA counts below 500, number of genes less than 200 or more than 2,500, and mitochondrial RNA content above 1% (as for single-nucleus sequencing, we expected none). Resulting peaks were also used for filtering of low-quality cells. Applied thresholds were: ATAC counts below 1,000, nucleosome signal more than 2 and transcription start site enrichment less than 1. In addition, peaks were removed that overlapped with non-standard chromosomes: genomic blacklist regions. The preprocessing led to 8,197 total cells for CDMCDLCD and 5,017 cells for HFDMCDLCD. Each RNA-seq dataset was pre-processed within each condition before integration using SCTransform, regressing out the percentage of mitochondrial DNA. The integration of RNA and ATAC data across conditions followed the outline from Kim et al.75. Accordingly, RNA data integration began with the selection of 3,000 integration features using SelectIntegrationFeatures in Seurat. The datasets were prepared for integration with PrepSCTIntegration, and integration anchors were identified using FindIntegrationAnchors with SCTransform (SCT) normalization. The anchors were then used to integrate the datasets across conditions via IntegrateData. ATAC count integration started by creating a feature matrix for each condition based on a unified peak set that was shared across conditions. Dimensionality reduction for ATAC data relied on latent semantic indexing, which included feature selection of most variable peaks, transformation of peak counts and singular value decomposition. To improve the integration, Harmony was applied (RunHarmony). Harmony aligns datasets by projecting them into a shared low-dimensional space while minimizing the effects of batch-specific biases. For the final integration across modalities, multimodal neighbours were identified by combining RNA-based PCA and ATAC-based Harmony embeddings (FindMultiModalNeighbors) across 50 dimensions for each modality, without the first dimension for Harmony, as commonly done, aiming for additional batch correction. Clusters were identified using the weighted nearest neighbour similarity graph (FindClusters) with a resolution of 0.5. This approach allowed the simultaneous integration of RNA and ATAC data for both conditions while leveraging the complementary information from both modalities. Cluster-specific marker genes were identified by calculating differential expression for each cluster using FindAllMarkers in Seurat. Markers were filtered based on minimum expression percentage (0.10), minimum differential expression percentage (0.20) and log fold-change threshold (0.20), retaining only positive markers. Cell-type annotation was based on the expression of a curated gene set within the identified markers. Subclustering was performed on the cells annotated as KCs from the integrated dataset. Again, multimodal neighbours were identified (FindMultiModalNeighbors) using PCA for RNA and Harmony for ATAC, followed by UMAP for dimensionality reduction (RunUMAP) and clustering (FindClusters), as done for the dataset using all cell types. Subclusters with fewer than 50 cells were excluded due to low numbers, and markers for each remaining cluster were characterized using the FindAllMarkers function in Seurat with the number of neighbours set to 60. Subcluster characterization was based on the expression of a curated gene set within the identified markers. To analyse intercellular communication involving the differing KC subclusters to hepatocytes, a CellChat analysis was performed. The analysis followed CellChat instructions for comparisons and analysis of multiple datasets. Normalized RNA expression data were used to define communication networks between KC subclusters and other cell populations. Overexpressed genes and interactions were identified for each condition, identifying 1,366 (CDMCDLCD) and 1,458 (HFDMCDLCD) highly variable ligand–receptor pairs subsequently used for signalling inference. Communication probabilities were computed using the 10% truncated mean for calculating the gene expression per cell group. Interactions with fewer than ten participating cells were filtered out to ensure robustness. The KC-specific communication networks were compared across conditions using rankNet function. A paired Wilcoxon test was performed to determine whether there was a significant difference of the signalling information flow between two conditions. Potential activated transcription factors were investigated using the package decouplR. For the analysis, the transformed RNA-count data were subsetted to the union of DEGs between the KC subclusters. The resulting set was subjected to the unified linear model to identify potential regulators (transcription factors). Only regulators were tested, which had at least ten known targets among the union differentially expressed set. According to decouplR’s vignette, the resulting score matrix, representing regulations strength, was scaled and centred. The 25% trimmed mean activity per cluster for each condition is displayed to provide a robust central tendency measure.
Metabolomics analysis
Metabolomics analysis was conducted using data obtained from Metabolon. The Metabolon platform performed preprocessing steps on the data, including imputation of missing values and natural log transformation. In summary, the raw peak area data were median scaled per metabolite and then normalized by the value of the extracted volume. It was further rescaled to have a median equal to 1. Missing values for each metabolite were imputed with the minimum value observed across all samples. Finally, a natural logarithm transformation was applied to the metabolomic data, as it typically exhibited a log-normal distribution. For the PCA, the data were subjected to batch correction based on the day of the experiment, which was identified as a significant source of variance in the initial PCA analysis. The identified effect was treated as a batch factor in the ComBat function of the sva package76 for batch correction. The corrected data were then used for PCA analysis. To identify metabolites with significant effects of diet, genotypes or their interaction on their respective levels, an ANOVA was performed. Specifically, a contrast77 was applied within the ANOVA to compare metabolite levels between maternal obese (HFDMCDLCD) and maternal lean (CDMCDLCD) conditions. The resulting set of 129 metabolites with significant raw P values was further analysed using Metaboanalyst (https://www.metaboanalyst.ca/)78 for pathway over-representation analysis. The HMDB IDs provided by Metabolon were used as input, resulting in a reduction to 111 metabolites due to missing labels. Furthermore, mapping to Metaboanalyst failed for ten additional metabolites. Default parameters were used for all remaining settings, except for choosing Mus musculus (KEGG) as the pathway library and applying out-degree centrality as the topology analysis method.
Hepatocyte and KC isolation for ex vivo experiments
Eight-to-ten-week-old chow diet-fed C57BL/6JRcc female mice were used to isolate hepatocytes. Therefore, a two-step collagenase liver perfusion was performed. First, 7 min after injecting heparin-sodium (Ratiopharm; 30 units per gram body weight), mice were anaesthetized with ketamine–xylazine. Next, a 26 G indwelling venous catheter (0.62 mm × 19 mm; 391379, BD Neoflon Pro) was inserted into the portal vein. Perfusion was initiated with 25 ml Hank’s balanced salt solution containing 0.5 mM EGTA (pH 7.4) at a rate of 2.5 ml min−1 using a peristaltic pump, and subsequently with 25 ml collagenase buffer (William’s Medium E, P04-29510, Pan-Biotech) supplemented with 3.6 mM CaCl2, 0.1 U ml−1 collagenase NB46 (17465, Serva Electrophoresis) and 10 mM HEPES at 37 °C at a rate of 1.25 ml min−1. Liver cells were released by gentle swirling with forceps into 40 ml suspension medium (William’s Medium E supplemented with 10% FCS, 2 mM l-glutamine, 100 units per millilitre penicillin and 100 µg ml−1 streptomycin). Hepatocytes were pelleted at 50g for 3 min at room temperature. The pellet containing the hepatocytes was resuspended in fresh suspension medium and plated on collagen-coated 24-well plates for co-culture experiments with KCs at a density of 30,000 cells per well or on collagen-coated 96-well plates for factor screening experiments at a density of 5,000 cells per well. To ensure a viable hepatocyte culture, cells were pre-incubated for 3 h at 37 °C and 5% CO2 to allow for attachment. Thereafter, the medium was replaced with fresh suspension medium containing LD540 (0.02 µg ml−1) and vitamin C (1 µg ml−1) for live-cell imaging using the IncuCyte SX5 (Sartorius). To isolate KCs, 11–15-week-old mice were perfused with the same method as isolating hepatocytes but without the in vivo digestion step. After perfusion, liver was minced and digested in a digestion mix (PBS containing 1 mg ml−1 collagenase D, 100 U ml−1 DNase I, 2.4 mg ml−1 dispase and 3% FCS) for 30 min at 37 °C without agitation. Digested liver was further disrupted with gentle pipetting, filtered through a 100-µm cell strainer and centrifuged at 400g for 10 min at 4 °C. Pellet was washed with ice-cold FACS buffer (PBS containing 0.5% BSA and 2 mM EDTA) and centrifuged at 50g for 3 min at 4 °C to remove hepatocytes. The supernatant was then further filtered and washed with FACS buffer to obtain a clean, single-cell suspension and centrifuged at 400g for 10 min at 4 °C. The resulting pellet was then blocked with 1% anti-mouse CD16/32 (101302, BioLegend) and 5% rat serum (C13SDZ, Bio-Rad) for 10 min at 4 °C followed by incubation with biotinylated CD11b antibody (101204, BioLegend) for 30 min at 4 °C. Cells were then washed, incubated with pre-washed streptavidin-conjugated magnetic beads (480016, BioLegend), washed and enriched in a 12-well untreated cell-culture dish mounted atop a magnet. Enriched cells were abundantly washed in FACS buffer and subsequently counted using a Neubauer chamber. Cells (3 × 105) were plated onto a 13-mm diameter, untreated glass coverslip (VWR; co-culture experiments) or 2–4 × 106 cells onto an untreated glass slide (Fisher Scientific; proteomics experiments). Cells were attached for 2 h in macrophage medium (DMEM, containing 1% penicillin–streptomycin, 10% FBS and 1 mM sodium pyruvate) in a cell culture incubator at 37 °C with 5% CO2. After attachment, cells were abundantly washed with PBS. Macrophages on coverslips were used for co-culture experiments as described below. Macrophages for proteomics experiments were scraped off in PBS, centrifuged at 400g at 4 °C for 5 min to obtain pellets, snap-frozen in liquid nitrogen and further processed for proteomics as described below.
Live-cell imaging for hepatocyte culture experiments and data analysis
For hepatocyte–KC co-culture experiments, a plastic ring (8 mm in height × 15 mm in diameter) was placed in each well of hepatocytes seeded as described above. A glass coverslip attached with approximately 3 × 104 KCs (resulted from initially seeded 3 × 105 CD11b magnet-enriched cells), isolated from either CDMCDLCD or HFDMCDLCD mice, was positioned upside-down on the plastic ring. LD540 was then added, and signal was recorded by live imaging hourly for 4 h in the IncuCyte SX5, with each mouse represented by multiple replicates and nine fields of view (FOVs) per well. For treatment of hepatocytes with APOE or APOA1, APOE (1 µg ml−1 recombinant mouse APOE; ab226314, Abcam) or APOA1 (2 µg ml−1 recombinant mouse APOA1; 50918-M08H, Sino Biological) was pre-incubated with hepatocyte medium at 37 °C for 30 min. The pre-incubated medium was then added to hepatocytes already containing 1 volume of hepatocyte medium. TNF (0.1 µg ml−1 recombinant mouse TNF; 130-101-688, Miltenyi)-treated hepatocytes were used as positive controls. Non-treated hepatocytes served as negative controls. For each mouse, three replicates were prepared, LD540 was then added and the signal was recorded by live imaging hourly for 4 h in the IncuCyte SX5, with four FOVs imaged per well. The LD540-integrated intensity was quantified by multiplying µm2 per FOV using the IncuCyte SX5 analysis software. A cell area filter was applied to ensure single-cell detection. Out-of-focus FOVs were excluded from analysis, as indicated by red labelling in the raw data spreadsheet. For co-culture data, the mean integrated LD540 signal per mouse was calculated following outlier removal by the interquartile range method. Statistical tests included the Shapiro–Wilk test for normality, Levene’s test for variance homogeneity, and two-sample t-tests to compare CD versus HFD conditions at each time point. For screening data, mean LD540 measurements were averaged across replicates and normalized to the control measurement mean for each mouse after 3 h and 4 h in the co-culture experiment, and after 2 h in the screening assay.
KC proteomics analysis
KCs were isolated and pelleted as described above. Pelleted cells were thawed and lysed in urea lysis buffer (8 M urea and 50 mM Tris (pH8)), DNA was sheared with 28 U ml−1 benzonase (Sigma), and disulfide bridges were reduced and alkylated with 10 mM Bond-Breaker TCEP solution (Thermo) and 30 mM chloroacetamide. Non-dissolved cellular debris was removed by centrifugation at 15,000g for 10 min, and clean supernatants were transferred to fresh tubes. Protein concentrations were determined with the Pierce 660 nm Protein-Assay-kit (Thermo). Of protein input, 25 µg was used for digestion in 2 M urea (diluted with 50 mM Tris (pH 8)) with a trypsin–LysC mixture (1:50 (enzyme:protein)) at 800 rpm for 16 h. Protein digestion was stopped with 1% formic acid, peptides were desalted on in-house-produced SDB-RPS Stage Tips and loaded onto EvoTip Pure trap columns following the manufacturers’ protocol.
A liquid chromatography–tandem mass spectrometry system consisting of an Evosep One liquid chromatograph (Evosep) and a timsTOF Pro 2 mass spectrometer (Bruker) was used for peptide separation and acquisition. Reversed-phase separation was performed on an Aurora Elite analytical column (IonOpticks) using the 40 samples-per-day separation method with a 31-min gradient preprogrammed on the liquid chromatography system. Eluting peptides were on-line transferred into the mass spectrometry via a CaptiveSpray ionisation source operated at a constant voltage of 1.5 kV. We used the data-independent acquisition parallel accumulation-serial fragmentation mode with the TIMS analyser operating with 100-ms accumulation and ramping times and a 100% duty cycle. Peptide ranges were limited to a mass-to-charge range of 350–1,100 m/z and an ion mobility range of 0.63–1.45 Vs cm−2 with 12 pydiAID-optimized data-independent acquisition parallel accumulation-serial fragmentation windows of variable widths79. We increased collisional energies stepwise from 24 eV at 0.70 Vs cm−2 and 49 eV at 1.35 Vs cm−2 for peptide fragmentation. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository80 with the dataset identifier PXD058285. Spectral library prediction, peptide identification and protein quantification were performed in library-free mode using DIA-NN (v1.8.1)81. The SwissProt Mus musculus database was used for predicted spectral library (downloaded from UniProt: 2022-12-28). In silico digestion was set to trypsin as the digestion enzyme with a maximal one miss-cleavage and cysteine carbamidomethylation set as fixed modifications. Precursor peptides were filtered at a false discovery rate (FDR) < 1%. The match-between-runs option was enabled. Using the DIA-NN main output table, we performed protein group intensity normalizations with the MaxLFQ algorithm82 implemented in the DIA-NN R-package. Only proteotypic peptides were considered for quantification. Statistical analysis was performed in the Perseus software suite (v1.6.15)83. Protein group label-free quantification intensities were log2 transformed and filtered for protein groups with data completeness in at least one condition. Missing values were replaced by random value drawing from 1.8 standard deviations downshifted and 0.3 standard deviations broad normal distributions. Significantly regulated proteins between any conditions were identified by ANOVA multiple-sample testing (S0 = 0.5, FDR < 0.1 with 250 randomizations) and all proteins were z-score normalized. For visualization, the dataset was summarized by taking the mean over all samples per group, namely, WT and KO in maternal lean, as well as WT and KO in maternal obese. The summarized data were objected to k-means clustering using k = 6 to obtain groups of proteins with varying expression patterns across samples from which several interesting representatives were chosen to be highlighted.
Statistics and bioinformatic tools
Statistical assessment was performed with one-way ANOVA with Tukey’s multiple comparisons method, the Kruskal–Wallis test, the unpaired Student’s t-test, the Mann–Whitney test or the Wilcoxon rank sum test, depending on the dataset, and following an assessment of normality. R-studio and Prism were used for the statistical evaluation and visualization. Statistical significance was represented via the probability (P value) as follows: not significant (NS) > 0.05, *P < 0.05, **P < 0.01 and ***P < 0.001. All bar graphs represent mean values. In all of the aforementioned analyses, PCA was performed using the prcomp function in R on centred data. Significance levels were consistently set at P < 0.05. Raw P values were adjusted for multiple testing using the false discovery rate method and considered significant if the adjusted P < 0.1. The analysis was implemented using R (v4.2.0)84 and Bioconductor (v3.15)85. Detailed information about every package used, including all dependencies, along with their respective versions, can be found in the renv.lock file located in the GitHub repository (https://github.com/LeaSeep/MaternalObesity) generated with the renv package86. The main analysis and visualization packages used can be found in Supplementary Table 9 that contains all references. All scripts, as well as the necessary data files, are available in the GitHub repository to reproduce all the results presented in the article, including additional results, by running the respective main files or provided RMarkdown Documents for the hCoCena. Furthermore, a database file has been provided, which presents all the statistical figures and serves as a comprehensive resource for quick reference and independent verification of our own hypothesis or results. These resources have been made available to facilitate reproducibility and enable researchers to conduct their own investigations with ease. A snapshot of the GitHub repository can be found on Zenodo87 (https://doi.org/10.5281/zenodo.14287647).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.