Human CSF
Patients with cancer undergoing routine clinical procedures including spinal tap, Ommaya reservoir tap or a ventricular shunt provided informed consent. CSF collected in excess of that needed for clinical care was reserved for this use under MSKCC Institutional Review Board-approved protocols 20-117, 18-505, 13-039, 12-245 and 06-107. Human CSF was processed as described previously6, de-identified and aliquoted. Cell-free CSF and CSF cell pellets were biobanked and stored at −80 °C until further analysis. Patient medical records and MRI scans were reviewed to confirm the LM status by neuro-oncologists (U.T.S., J.A.W. and A.B.), and clinical data necessary for this study were abstracted and de-identified by neuro-oncologists or clinical research assistants (R.E. and K.C.). Giemsa-stained cytospins were part of routine diagnostic assessment and were retrieved and reviewed by a neuropathologist (T.A.B.).
Human single-cell transcriptomics
Sample processing
Freshly collected CSF obtained by lumbar puncture was placed on ice and processed within 2 h, as described previously6. PBS-washed cells were encapsulated using the Chromium Single Cell 3’ Library and Gel Bead Kit V2 (10x Genomics) and sequenced on the NovaSeq 6000 system (Illumina). Raw and preprocessed data were deposited to the NCBI GEO under accession number GSE221522.
Data preprocessing, initial processing and batch correction
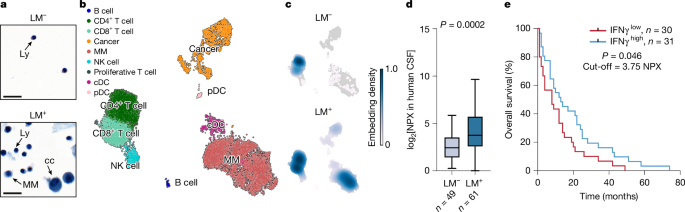
Raw FASTQC files were pre-processed with SEQC52,53 with human reference genome hg38, and dense SEQC matrices were imported into Python. Each sample was plotted as a histogram of total counts per cell barcode on the log scale, resulting in a distribution with multiple modes and the threshold to remove the smallest mode, containing empty droplets and low-quality cells, was defined manually. We next removed any genes that had counts equal to 0 after filtering. To remove doublets, we run the DoubletDetection method (parameters n_iter = 50, p_thres = 1e-7, voter_thres = 0.8)54. We outer joined the individual samples to retain all of the detected genes, filtered cells to a minimum count of UMI = 100 and minimum total expressed genes of 100. We initially detected 22,051 cells and retained 20,676 high-quality cells and 18,322 genes after filtering. We detected around 1,497 ± 898 genes per cell, about 6,268 ± 6,553 gene counts per cell, out of which 3.25 ± 2.99% were mitochondrial genes (values represent mean ± 1 s.d.). We normalized the library size, keeping the raw count matrix for downstream analyses and removed any genes expressed in fewer than five cells. For downstream analysis, we further removed mitochondrial genes (prefix MT-), ribosomal genes (prefix RPS- or RPL-) and haemoglobin genes (prefix HB-). We run Scanorama (default settings; knn = 20)55 on the resulting AnnData object to batch correct across patients. Batch correction was validated as follows: (1) cancer cells have higher interpatient heterogeneity (Supplementary Fig. 1), suggesting the absence of overcorrection; and (2) we identified and filtered out only few quasi-cancer cells from patients without LM after computational mixing (their presence was ruled out by pathologist during diagnostic cytology reading). This corrected matrix was used for visualization, but not for individual gene comparisons. We then run PCA (sc.pp.pca, n_components = 100). We constructed a k-nearest neighbour graph (k-NN) based on the 30 nearest neighbours and 100 principal components, using the Scanorama 100-dimensional matrix (instead of PCA matrix). We clustered the cells with Leiden clustering (resolution 2.0)56 and these Leiden clusters were merged according to major cell types, which were assigned on the basis of marker gene expression, as shown in Supplementary Fig. 1. The UMAP was computed with sc.tl.umap, using the default parameters. The interpatient heterogeneity was measured with Shannon entropy, Hj (ref. 57) (Supplementary Fig. 1g–i):
$${H}_{j}=\sum _{T}-\,{q}_{T}\log {q}_{T}$$
For each cell, the Shannon entropy measures the sample diversity of its nearest neighbours in the k-NN graph. Each sample was subsampled to contain 500 cells. If samples are well-mixed, the entropy of each cell will be high while, if samples are not well mixed, the entropies will tend to be low (this is true for cancer cells in general, which show extreme heterogeneity across patients). Quality-control plots are shown in Supplementary Fig. 1. Human LM+ single-cell transcriptomic data was retrieved from NCBI GEO GSE150660 (ref. 6). Raw and pre-processed data are available at the NCBI GEO under accession number GSE221522. All ten human samples were collected between December 2017 and May 2018 and were processed with the same pipeline.
Subsetting of cells for downstream analyses and visualization
Subsetting was performed by selecting cell clusters from major Leiden populations, shown in Fig. 1b. For analysis of DCs, the cDC and pDC clusters were subsetted and reclustered with sc.tl.umap and Leiden (resolution = 0.5). Cell type annotation was performed as follows: cDC1 cells are CLEC9A+XCR1+, cDC2 cells are CLEC10A+CD1C+, pDC cells are IRF7+TCF4+. Human LAMP3+ migratory DCs are LAMP3+CCR7+ (orthologous to mouse CCR7+ DCs; Supplementary Fig. 4). Two clusters bearing cDC2 signature were merged for further analyses. For analysis of NK, NK cluster was subsetted and reclustered with Leiden (resolution = 0.8), yielding in populations of cells with high SELL (CD62L) expression, further denoted as naive-like, and populations with low SELL expression, denoted as activated-like and characterized by the expression of CXCR6. For analysis of both cell types, we run Palantir with the default settings (n_components = 5, knn = 30), enabling us to access Markov affinity-based graph imputation of cells (MAGIC-imputed) cell counts, and these imputed cell counts were used only for visualization with 2D plots35,58. UMAP, t-SNE and heat-map plotting was performed using the Scanpy59 and scVelo60 toolkits. Embedding density was computed with sc.tl.embedding_density (Fig. 1c). For NK cell gene expression heat map, counts were first zero-centred with sc.pp.scale (Supplementary Fig. 5).
Gene signature analysis
Gene signatures were computed with the Scanpy function sc.tl.score_genes and projected onto the UMAP (Fig. 1b) and matrixplot. IFNγ gene signature 1 was created based on our mouse dataset, comparing the gene ranks in all cells from eGFP versus Ifng cancer-bearing mice to mimic the situation in our patients. Genes were ranked with sc.tl.rank_genes_groups, t-test method. This gene set contained the following genes: S100A9, S100A8, IRF7, PLAC8, ALOX5AP, FGL2, ISG15, CD74, PSAP, NAPSA, SLFN5, TALDO1, PLBD1, SPI1, MPEG1, LCN2, NAAA, TRIM30A, IRF8, NCF1, CBFA2T3, NGP, MS4A4C, CHIL3, CYBB, SLAMF7, RNASE6, RETNLG, CLEC12A, IFI205, CAMP, SLFN1, CKB, IGKC, IFIT3, AMICA1, LY86, OASL2, CSF2RA, CD24A, XCR1, OAS3, IFITM6, CLEC9A, IFIT2, LTF, WDFY4, LY6I, WFDC21 and USP18 (50 genes after removal of mouse-specific genes, including HLA). IFNγ gene signature 2 GSEA Hallmark interferon gamma response gene set was retrieved online (https://www.gsea-msigdb.org/gsea/msigdb/human/geneset/; 200 genes).
Human CSF targeted proteomics
The samples were processed and analysed essentially as described previously9. Biobanked CSF collected between 2015–2020 was aliquoted and stored at −80 °C at MSK Brain Tumor Center CSF Bank. Samples were slowly thawed on ice and 45 μl of CSF was mixed with 5 μl of 10% Triton X-100 (Sigma-Aldrich, T8787) in saline and incubated at room temperature for 2 h (final concentration of Triton X-100 was 1%). The samples were then dispensed in a randomized manner into 96-well PCR plates and stored at −80 °C until further analysis. Relative levels of proteins in two targeted panels were detected using proximity extension assay (Olink Target 96 Inflammation and Olink Target 96 Neuro Exploratory, Olink). As an additional control, LM− samples were retrieved from a previous study9 (CoV− cohort). Protein abundance values are shown in NPX units (on the log2 scale). The analytical range for each analyte is available online (www.olink.com).
Mouse strains and housing
All animal studies were approved by the MSKCC Institutional Animal Care and Use Committee under the protocol 18-01-002. WT C57BL/6 (JAX, 000664) were purchased from Jackson Laboratory or bred in-house. C57BL/6-Tyrc-2 (JAX, 000058, albino C57Bl/6) and BALB/c (JAX, 000651) animals were purchased from the Jackson Laboratory. NSG animals were obtained from MSKCC RARC Colony Management Group. Purchased mice were allowed to habituate for at least 1 week before manipulation and experimentation. Transgenic lines on the C57BL/6 background were purchased from the Jackson Laboratory and bred in house: Ifng-knockout line (B6.129S7-Ifngtm1Ts/J, JAX, 002287)15, Ifngr1-knockout line (B6.129S7-Ifngr1tm1Agt/J, JAX, 003288)16, Rag1 knockout line (B6.129S7-Rag1tm1Mom/J, JAX, 002216)61, double-reported knock-in/knockout Cx3cr1GFP/GFPCcr2RFP/RFP line (B6.129(Cg)-Cx3cr1tm1LittCcr2tm2.1Ifc/JernJ, JAX, 032127)62,63, congenic B6 CD45.1 line (B6.SJL-PtprcaPepcb/BoyJ, JAX, 002014), Zbtb46-DTR line (B6(Cg)-Zbtb46tm1(HBEGF)Mnz/J, JAX, 019506)27. For homozygous breeding, breeding pairs and randomly selected progenies used in the experiments were genotyped as recommended. For experiments that involved bioluminescence imaging where WT animals were not compared to transgenic lines, albino C57BL/6-Tyrc-2J animals were used. Mice in all experimental groups were age (±4 days), sex and fur-colour matched. Mice used in this study were housed under specific-pathogen-free conditions, in an environment with controlled temperature and humidity, under 12 h–12 h light–dark cycles (lights on and off at 06:00 and 18:00, respectively), and with access to regular chow and sterilized tap water ad libitum.
Compound transgenic mouse lines
We used the following alleles on C57BL/6 background to generate the compound strains: Clec9acre (C. Reis e Sousa)30, Ifngfl/fl (A. Rudensky), Ifngr1fl/fl (JAX, 025394)64, Lckcre (JAX, 003802)65, LysMcre (JAX, 004781)66, Nkp46cre (E. Vivier)67, Rosa26lsl-DTR (JAX, 007900)68, Rosa26lsl-eYFP (JAX, 007903)69, Xcr1cre-mCherry (JAX, 035435)70. To generate the Clec9acreRosa26lsl-DTR depletor line, heterozygous Clec9acreRosa26lsl-DTR mice (Brown laboratory) were crossed together. To generate the conditional NK cell Ifng-knockout line, heterozygous Nkp46creIfngwt/fl animals (Sun laboratory) were crossed to establish the Nkp46creIfngfl/fl colony. To generate the conditional T cell Ifng-knockout line T cellΔIfng, Nkp46creIfngwt/fl mice were crossed to Lckcre animals. The Nkp46cre allele was bred out and LckcreIfngfl/fl line was established. To generate the conditional myeloid cell Ifngr1-knockout line, LysMcre animals were crossed with Ifngr1fl/fl mice to establish the LysMcreIfngr1fl/fl line. To generate the conditional cDC Ifngr1-knockout line, heterozygous Clec9acreRosa26lsl-DTR animals (Brown laboratory) were crossed with Ifngr1fl/fl mice and the Rosa26lsl-DTR allele was bred out to establish the Clec9acreIfngr1fl/fl line. Similarly, to generate the conditional NK-cell Ifngr1-knockout line, heterozygous Nkp46creIfngwt/fl animals were crossed with Ifngr1fl/fl mice and the Ifngwt/fl allele was bred out to establish the Nkp46creIfngr1fl/fl line. Progeny was genotyped and the cre-negative littermates on homozygous flox/flox were used as controls. For DC lineage tracing, the homozygous Xcr1cre-mCherry line was crossed with the homozygous Rosa26lsl-eYFP line. Mice were housed as described above.
Cell culture
Mouse lung cancer LLC sublines were described previously3. Mouse breast cancer E0771 cells were a gift from E. E. Er. B16-F10 (CRL-6475), Yumm5.2 (CRL-3367), EMT6 (CRL-2755) and 4T1 cells (CRL-2539) were obtained from ATCC. LentiX 293T cells (632180) were obtained from Takara. PlasmoTest HEK Blue-2 cells (rep-pt1) were obtained from Invivogen. LLC, E0771 and B16 sublines and LentiX 293T and HEK Blue-2 cells were maintained in high-glucose DME (MSKCC Media Core), supplemented with 10% fetal bovine serum (FBS; Omega Scientific, FB-01) and 1% penicillin–streptomycin (Gibco, 15140163) or 1× Primocin (Invivogen, ant-pm-2). Yumm5.2 sublines were maintained in high-glucose DME:F12 (MSKCC Media Core), supplemented with 10% FBS, 1% non-essential amino acids (Gibco, 11140050) and 1% penicillin–streptomycin or 1× Primocin. 4T1 sublines were maintained in RPMI (MSKCC Media Core), supplemented with 10% FBS and 1% penicillin–streptomycin or 1× Primocin. EMT6 sublines were maintained in Waymouth’s (MSKCC Media Core), supplemented with 10% FBS and 1% penicillin–streptomycin or 1× Primocin. Cell lines were subcultured at least twice a week, replaced approximately after 6 weeks in culture with new stocks, stored in liquid nitrogen and routinely tested negative for mycoplasma contamination. Cell lines that were not obtained directly from ATCC (LLC, E0771) were authenticated with STR profiling (ATCC). Proliferation of vehicle-exposed or recombinant mouse IFNγ-exposed (BioLegend, 714006) cancer cells in vitro was measured with CellTiter-Glo luminescent cell viability assay (Promega, G7572) 72 h after seeding 500 cells per well into 96-well, white-walled plate (Corning).
Genetic engineering of mouse cancer cell lines
Plasmid DNA was amplified in NEB stable competent Escherichia coli (New England Biolabs, c3040i) or other E. coli strains provided by vendors, grown in LB broth (MSKCC Media Core) overnight and isolated with ZymoPURE II kit (Zymo Research, D4203). Mouse cancer cell lines generated in this study were engineered to constitutively express V5-tagged Firefly luciferase (pLenti-PGK-V5-Luc-Purow543-1, Addgene, 19360), gift from E. Campeau and P. Kaufman. Some LeptoM derivatives (LLC LeptoM, E0771 LeptoM, B16 LeptoM) used in the flow cytometry experiments were additionally engineered to constitutively express AmCyan fluorescent protein (pLV-EF1a-AmCyan1-IRES-Puro, Takara, 0039VCT). Lentiviral constructs for CRISPR–Cas9 editing in the pLV-hCas9:T2A:Bsd backbone were synthetized by VectorBuilder. sgRNA sequences expressed under the control of U6 promoter were as follows: sglacz, TGCGAATACGCCCACGCGAT; sgIfngr2.1, TGGACCTCCGAAAAACATCT; sgIfngr2.2, AGGGAACCTCACTTCCAAGT; sgIfngr2.3, TCTGTGATGTCCGTACAGTT. Lentiviral particles were prepared with LentiX 293T cell line using ecotropic, VSV-G pseudotyped lentiviral system and concentrator (Takara, 631276 and 631232), as recommended. Mouse cancer cell lines were spin-transduced (1,000g, 32 °C, 1 h) with concentrated lentiviral particles in complete culture medium containing 5 μg ml−1 hexadimethrine bromide (Santa Cruz, sc-134220) and selected for 5–7 days in complete medium containing 2–5 μg ml−1 puromycin (Gibco, A1113802) or 5–10 μg ml−1 blasticidin (Invivogen, ant-bl-1). CRISPR–Cas9 edited lines and control clones were single-cell sorted into 96-well plate. Gene function was assessed functionally (LLC, E0771 and B16 LeptoM; Extended Data Fig. 7), and DNA editing was confirmed with Sanger sequencing (LLC and E0771 LeptoM; not shown) after expansion.
Cancer cell injections
Cancer cells were injected into mice between 6 and 16 weeks of age. Mice were deeply anaesthetized in an insulated chamber perfused with 2–3% isoflurane (Covetrus, 11695067772) in medical air or with intraperitoneally delivered mixture of ketamine (100 mg per kg) and xylazine (10 mg per kg) in ultra-pure, sterile and pyrogen-free water for injection. Female mice were used for breast cancer models and both males and females in approximately 1:1 ratio for melanoma and lung cancer models, if not stated otherwise. Mice deceased within 72 h of injection were excluded from further analysis. Mouse hair was removed from the injection site, and the area was sterilized three times with ethanol. For intracisternal injection, 10 μl of cancer cell suspension in PBS was introduced into the cisterna magna using Hamilton syringe (Hamilton, HT80501) fitted with a 30 G needle, as described previously with minor modifications3. In brief, the mouse was positioned prone over a 15 ml conical tube to place cervical spine in flexion. The occiput was palpated, the needle was advanced 4 mm deep and the syringe content was slowly released into the cisterna magna. The syringe was then held in this position for another ten seconds and then carefully ejected to prevent the reflux of injected liquid. This procedure was tolerated well by the animals (success and survival rate > 95%). Mice displaying neurological symptoms after awakening were immediately euthanized. The number of cancer cells introduced intracisternally was as follows: 2,000 cells for LLC LeptoM; 4,000 cells for E0771 LeptoM; and 500 cells for B16 LeptoM, Yumm5.2 LeptoM, EMT6 LeptoM and 4T1 LeptoM cells. For intracardiac injections, 10,000 cells (for 4T1 or EMT6 sublines) or 50,000 cells (all other sublines) were injected in 50 μl saline using a 28-G insulin syringe into the left cardiac ventricle. For extracranial injections, cells were injected in 50 μl percutaneously into the fourth mammary fat pad (E0771, LeptoM; 500,000 cells), subcutaneously (LLC LeptoM; 200,000 cells) or intradermally (B16 LeptoM; 100,000 cells) using a 28-G insulin syringe. For primary tumour priming experiments (Extended Data Fig. 3), selected cell sublines were first injected into the extracranial sites. Then, 2 weeks later, mice were challenged with the same cancer cell sublines delivered intracisternally. Non-imaged tumours were shielded with a sheet of black watercolour paper to prevent capture of scattered photons. Images for cranium and extracranial site were therefore captured separately in the same mouse.
Quantification of tumour burden
The spread and growth of cancer cell lines engineered to express V5-tagged Firefly luciferase (lucV5) was monitored using non-invasive bioluminescence imaging (BLI). Mice were anaesthetized in an insulated chamber perfused with 2–3% isoflurane in medical air and injected retro-orbitally with 50 μl of sterile d-luciferin (15 mg ml−1, Goldbio, LUCK-5G) solution in PBS. BLI was captured using IVIS Spectrum-CT (Perkin Elmer). Data were recorded and processed using Living Image (v.4.7.2) software. Recorded images were quantified as cranial radiance. On the rare occasion when mice on the C57BL/6 background (without tyrosinase mutation) developed melanin spots preventing luciferase imaging, these animals were not included in the imaging analysis. For tumours in the mammary fat pad, intradermal and subcutaneous tumours were measured using calibrated digital callipers (VWR, 62379-531) or BLI. Tumour volumes are expressed as the product of the two largest diameters, as described previously71. Imaged areas were shaved to expose the skin before the imaging. Dark-furred mice that developed benign pigmented skin lesions in the shaved areas that interfere with BLI were not included in tumour quantification. Euthanasia was indicated when the tumour outgrowth from the injection site exceeded 1 cm3 or in the event of distress, including disease-specific averse neurological symptoms in the mouse such as head tilt, limb weakness or paralysis, and lethargy, whichever occurred first. Mice were monitored daily for the occurrence of neurological symptoms, bioluminescent imaging was generally performed once a week, palpable tumours were measured, and sizes were calculated twice a week. No animals exceeded the limits approved by the MSKCC IACUC.
Quantification of leptomeningeal tumour burden with image analysis
B16 melanoma sublines growing in 3D structures produce high amounts melanin that quenches light in a wide spectrum of wavelengths, interfering with accurate bioluminescent and fluorescence imaging. For these tumours, bioluminescence was therefore used solely to confirm the presence or identify the anatomical location of cancer. To overcome this limitation and to accurately quantify the tumour burden in the B16 LeptoM model, the brains from intracisternally injected mice were dissected, preserving the plaques of cancer, and fixed in formalin overnight. The brains were then carefully washed with tap water and placed into six-well dishes in 70% ethanol. Bright-field images of fixed brains (basilar plane) were taken using Lumar Stereoscope (Zeiss) against dark background. Data were processed with Fiji/ImageJ (v.2.0.0, NIH) as follows: images were converted to 8-bit, each brain was manually encircled and its area was recorded. The threshold for plaque measurement was first estimated in a small cohort to capture only the plaque areas and then applied to all subsequent measurements. The percentage of the area of cancer plaques covering the basilar surface of the brain was calculated as the area of plaques divided by the area of brain and multiplied by 100. As the 8-bit images were monochromatic, this method showed to be robust and reproducible throughout different measurements. Five control brains from mice without cancer, collected for different purposes, were measured and the area of darker structures above the pre-set threshold was less than 1% using this method.
Derivation of leptomeningeal and parenchymal metastatic cell lines
BrM cell lines (brain parenchyma-tropic)
A total of 50,000 parental cells was injected intracardially. Hematogenous dissemination was confirmed using BLI approximately 1 h after injection. After confirmation of brain colonization with BLI and development of late-stage cancer symptoms, mice were reinjected with luciferin and euthanized. The brains were dissected and imaged ex vivo to confirm colonization of parenchyma. Brains with overt lesions were minced, mechanically dissociated using GentleMACS (Miltenyi Biotec) and digested in a mixture of collagenase (100 U ml−1, Worthington, LS005273) and DNase I (10 U ml−1, Worthington, LS006333) in HG DME for 1 h at 37 °C, mechanically dissociated every 20 min. The suspension was then washed, filtered through a 70 μm mesh and seeded into corresponding complete culture medium, in which penicillin–streptomycin was replaced with Primocin. The medium was changed every day for 3 days, then every other day. Growing cancer cell colonies were expanded for three passages and named BrM1. These cells were then again injected intracardially and the whole procedure was repeated, leading to the establishment of BrM2 cell lines, competent to colonize brain parenchyma after hematogenous dissemination.
LeptoM cell lines (leptomeninges-tropic)
A total of 2,000 lucV5-expressing parental cancer cells in 10 μl saline was injected intracisternally. The presence in the CSF was confirmed with BLI approximately 1 h after injection. Mice were monitored weekly using BLI and daily checked for the presence of pathophysiological symptoms. When these mice developed neurological symptoms (moribund behaviour, head tilt, seizures, overall weakness) and cancer presence in the CSF was indicated by BLI, luciferin was injected retro-orbitally and mice were euthanized. The brain was dissected as described above and basilar sides of brains as well as basilar meninges of mouse were assessed with BLI post mortem. The cranial cavity and brain surface were then washed with approximately 3 ml of saline. This volume was collected, pelleted, resuspended in complete medium containing Primocin and maintained as described above for BrM cells. This procedure was repeated once for melanomas or three times for epithelial cancers, leading to the establishment of Inter cell lines. These Inter cells were then injected intracardially and the mice were monitored with BLI and treated as described above. Successfully expanded cancer cells that were isolated from these intracardially injected mice had the ability to colonize the leptomeninges and grow in the CSF, and were therefore named LeptoM cells. Three to five biologically independent sublines were successfully established per cell line. For transcriptomic analyses, these replicates were processed separately retaining the ID of founder mice. For further in vitro and in vivo manipulations, these replicates were pooled (at one-to-one ratios) and maintained under subconfluent conditions in vitro for a limited number of passages (less than 12).
RNA collection and extraction and transcriptomic analysis
Cancer cell lines were collected 24 h after initial seeding of approximately 1 × 106 cells per 100 mm plate by direct lysis with RLT buffer (Qiagen, component of RNeasy kits). RNA from cell lines was isolated with RNeasy Plus Mini Kit (Qiagen, 74136), and sequenced and analysed as described previously72. Resulting HTSeq73 matrices from bulk transcriptome were processed in R Studio with DESeq274. Data from LLC cell lines were retrieved from NCBI GEO GSE83132. Newly generated raw and preprocessed data are available through NCBI GEO under accession number GSE221358.
Collection of mouse CSF and leptomeningeal immune cells
Mice were deeply anaesthetized using ketamine–xylazine and transcardially perfused with sterile, ice-cold PBS. Mice were positioned as described in the ‘Cancer cell injections’ section, and CSF was collected through the cisternal puncture into a PBS-flushed syringe fitted with a 30-G needle. Approximately 5–15 μl of CSF was collected from each single mouse using this procedure. Blood-contaminated samples were discarded. CSF was flash-frozen on dry ice and stored at −80 °C until analysis, or diluted in 200 μl of 4% methanol-free paraformaldehyde (Electron Microscopy Sciences, 15714-S) and spun onto microscopy slides to produce cytospins. These were then left to air dry and stained with haematoxylin QS (Vector Biolabs, H-3404-100). Leptomeningeal immune cells were collected as described previously6 and processed further for downstream applications, as described in the corresponding sections. Controls were prepared from mechanically dissociated spleens (GentleMACS, spleen program) and enzymatically dissociated brains and lungs (dissociation described above, mechanical assistance was performed with GentleMACS, brain and lung programs).
Intracisternal delivery of recombinant proteins and AAV particles
Vehicle (PBS) or a 10 ng or 25 ng dose of recombinant mouse IFNγ (BioLegend, 714006) in total volume of 10 μl was initially delivered with cancer cell injection, followed by weekly administration, as described above. Heat-inactivated IFNγ was prepared by incubating vehicle or vehicle-diluted IFNγ at 95 °C for 15 min and allowed to cool on ice before administration. Mouse Ifng (NM_008337.4) or eGFP sequences were inserted into AAV expression vector (pscAAV backbone under the control of CMV promoter) and used for packaging into AAV5 particles that were ultrapurified for in vivo applications (VectorBuilder). Genomic content was estimated with PCR. A total of 5 μl of vehicle-diluted AAV5 suspension (1 × 1013 genome copies per ml) was slowly infused into mouse leptomeninges intracisternally and mice were allowed to rest for at least 2 weeks before further manipulation.
Mouse single-cell proteogenomics
Sample processing
Cx3cr1GFP/GFPCcr2RFP/RFP mice were crossed with WT C56BL/6 mice and the resulting female and male Cx3cr1+/GFPCcr2+/RFP progeny were intracisternally infused with AAV and LLC LeptoM cancer cells, as described above and in Supplementary Fig. 3. Leptomeningeal cells from six animals per group were isolated and resuspended in Cell Staining Buffer (BioLegend, 420201). In total, we profiled leptomeningeal immune cells from 24 mice and 4 different conditions. To limit non-specific antibody binding, cells from each mouse were incubated with TruStain FcX (BioLegend, 101320), subsequently barcoded with TotalSeq-A anti-mouse hashtags 1 to 6 (BioLegend), listed in Supplementary Table 4, and washed. Cells from these six mice were then pooled, resulting in four independent samples, and stained with a custom TotalSeq-A panel (BioLegend), consisting of 198 antibodies targeting cell-surface epitopes and non-targeting isotype controls, listed in table Supplementary Table 5, to facilitate identification and origin of selected immune cell types (such as in Fig. 4). Dead cells and debris were removed with LeviCell (LevitasBio), and washed cells were counted, encapsulated using the Chromium Single Cell 3’ GEM Library and Gel Bead Kit V3.1 (10x Genomics) and sequenced on the NovaSeq 6000 system. Quality control plots are shown in Supplementary Fig. 3. Raw and preprocessed data are available through NCBI GEO under accession number GSE221593.
Data preprocessing and initial processing
Raw FASTQC files were preprocessed with SEQC52,53 with modified mouse reference genome mm10 that included GFP, RFP and AmCyan sequences, and preprocessed as human samples, with the exception that no batch correction was applied. Each sample was processed separately. Cell filtering and doublet removal with DoubletDetection (p_thresh=1e-16, voter_thresh=0.5, n_iters=25, use_phenograph=False, standard_scaling=True)54 was performed as described above for human samples, we initially detected 54,781 cells and 20,804 genes and retained 46,852 high-quality cells and 18,277 genes after filtering out low-quality cells and non-immune cell populations. We detected around 1,387 ± 866 genes per cell, about 4,374 ± 5,483 gene counts per cell, out of which 3.15 ± 2.62% were mitochondrial genes (values represent mean ± 1 s.d.). The Shannon entropy for this uncorrected mouse dataset was computed as described above for human data. AnnData files for each sample were then merged after filtering and doublet removal by an outer join. Erythrocyte genes (HBA-A1, HBB-BT, HBA-A2, HBB-BS, ALAS2, HBB-BT, HP and BPGM) and CD41 protein signal (platelet marker) were filtered out, in an addition to mitochondrial (prefix MT-) and ribosomal genes (prefix RPS- or RPL-). HTO and CITE-seq data were demultiplexed with cite-seq-count75, using the default parameters applied on the whitelist of cells that passed the filtering step based on RNA quality, as described above. RNA and protein data (HTO and CITE) were integrated with totalVI, facilitating identification of immune cell subtypes using both gene and surface protein expression (default settings with top 4,000 highly variable genes (HVGs))76. HTOs were assigned based on maximum number of observed counts (Supplementary Fig. 3e). UMAP k-NN graph and Leiden clustering56 in this dataset was computed using sc.pp.neighbours59 and totalVI processed latent variables. Leiden clusters were merged according to major cell types, which were assigned based on marker gene and surface protein expression, as showed in Supplementary Fig. 2 (HVGs).
Subsetting of cells for downstream analyses, plotting and visualization
Plotting was performed using Scanpy (UMAP, t-SNE, heat maps)59 and scVelo (UMAP, t-SNE; this package was not used to infer RNA velocity)60. Embedding density was computed with sc.tl.embedding_density (Fig. 2c). Cell cycle prediction was adapted from tl.score_genes_cell_cycle60 (Fig. 4j,k and Fig. 5c). Subsetting was performed by selecting cell clusters from major populations, shown in Fig. 2b. We included cells from all four conditions, shown in Supplementary Fig. 3: cells isolated from naive, vehicle-injected or LLC LeptoM-injected animals that were overexpressing eGFP (control gene) or Ifng specifically in the leptomeninges. For analysis of DCs, cDC and pDC clusters were subsetted, these cells were expressing CD11c (pan-DC marker) on cell surface. For analysis of NK cells, NK and proliferative T/NK clusters were subsetted to ensure proper representation of all NK cells. These cells were reclustered with Leiden (resolution = 0.7), and clusters expressing CD3 and TCRβ cell surface markers were excluded, retaining only bona fide NK cells, characterized as Nk1.1+CD3−TCRβ−. For analysis of both cell types, we run Palantir (default settings, n_components = 5, knn = 30) that allowed us to (1) compute diffusion components, used for t-SNE re-embeddings; and (2) access MAGIC-imputed cell counts35,58 (Figs. 4d and 5a,d). These imputed cell counts were used only for visualization with 2D plots. t-SNE plots were refitted using multiscale coordinates that are based on diffusion components obtained with Palantir (n_components = 5, knn = 30). Subsetted DCs were refitted onto t-SNE using Palantir multiscale coordinates and annotated with initial Leiden loadings to identify four typical DC populations. We considered both gene expression data (shown as a heat map in Supplementary Fig. 4a) and cell surface signals: cDC1 cells are XCR1+, cDC2 cells are CD11b+, pDC cells are B220+ and CCR7+ cells express CCR7 (Fig. 4e and Extended Data Fig. 12d). Subsetted NK cells were refitted onto t-SNE using Palantir multiscale coordinates and reclustered with Leiden (resolution = 0.3), resulting in the identification of four putative cell states. Naive NK cells expressed high cell surface levels of CD62L (encoded by SELL), while activated and proliferative cells had low CD62L levels. Proliferative cells also expressed genes associated with cell cycling, such as MKI67, TOP2A and HMGB2. Senescent cells expressed CD55 and KLGR1 on their cell surface (Fig. 5b). Cancer cells, characterized by the expression of keratin genes and CD63, were subsetted as a cancer cluster and were visualized with UMAP without re-embedding. Cancer cell gene signatures were computed with GSEApy (Extended Data Fig. 13j,k; cut-offs are provided in the corresponding figure legends) (https://github.com/zqfang/GSEApy).
Trajectory analysis
To predict the maturation trajectories of cDCs in normal, non-perturbed steady-state mouse leptomeninges and leptomeninges with metastasis, we subsetted CD11c+ cDCs from naive and cancer-bearing mice overexpressing eGFP only (cDC cluster and eGFP condition). We first used CellRank to identify putative trajectories without the need for initial or terminal state selection34. We filtered out genes present in less than ten cells, normalized counts per cell and with log(X + 1) and extracted HVGs with Scanpy’s functions sc.pp.filter_genes, sc.pp.normalize_total, sc.pp.log1p and sc.pp.highly_variable_genes. We retained 2,635 cells and 2,090 cDC-expressed HVGs. We recomputed PCA with sc.pp.pca (n_comps = 50, zero_centered = True) and refitted the t-SNE plot with the top nine diffusion components in multiscale space (n_components=9, knn=15); this t-SNE map was used for further visualization. We used cytoTRACE kernel77, which enabled us to assess plausible and biologically traceable cell transitions, following their trajectory from more primitive to mature cells. We imputed gene counts from normalized and filtered count matrix with scv.pp.moments with the default parameters (n_pcs = 30, n_neighbors = 30) and initialized CellRank’s cytoTRACEkernel with the default parameters. Transition matrix was computed (threshold_scheme = hard). Given that this approach provides qualitative insights into the transition matrix by iteratively choosing the next cell based on the current cell’s transition probabilities, we further compared two additional settings: (1) we did not specify from which cells or condition to select starting point (start_ixs = None); or (2) we selected all cells from naive eGFP-overexpressing mouse as the starting points. Both approaches identified CCR7+ DCs as mature end points, and to remain agnostic to the initiation, we continued the analysis without initial cells or states being defined (n_sim = 100). We used GPCCA estimator (generalized Perron cluster analysis)78 to coarse-grain a discrete Markov chain into a set of macrostates and compute coarse-grained transition probabilities among the macrostates. We identified three macrostates and assigned each cell their dominant microstate membership. These results suggested that the cDC2 population is prone to maturate towards CCR7+ DCs, with an insignificant contribution of the cDC1 population (Extended Data Fig. 12f–h). CellRank prediction was corroborated by an analysis using Palantir (n_components = 9, knn = 15, num_waypoints = 500)35 that identified the cDC2 population as the one with the highest entropy (maturation potential), and this observation was robust to changes in the number of diffusion components, neighbours or waypoints (Extended Data Fig. 12i,j). We dissected the cDC2-to-CCR7 DC transition axis and plotted smoothened gene trends along the predicted Palantir pseudotime axis (Fig. 4f).
Bone marrow chimeras
For DTR depletion experiments, male C57BL/6-Tyrc-2 mice were initially anaesthetized with 2–3% isoflurane in medical air and restrained in ventilated conical plastic tubes. Animals were placed in a prone position and irradiated using X-RAD320 irradiator (Precision) with the following settings: 250 kV; 12 mA; using 0.25 mm copper filter; distance of radiation source to the animal body, 50 cm; irradiation field, 20 × 20 cm; dose rate, 117.5 cGy min−1. Five mice were fitted into the radiation field and received and two cycles of 5.5 Gy total body radiation 6 h apart. Immediately after completion of the irradiation procedure, animals were returned to their cages and fed with sulfatrim-enriched diet for the duration of this experiment. Within 24 h, mice were retro-orbitally infused with approximately 1 × 107 bone marrow cells from multiple pooled WT or Zbtb46-DTR+/+ C57BL/6 donors. Bone marrow cells were sterilely isolated from femur and tibia. Inner bone marrow was exposed and placed inside a 0.6 ml PCR tube with small hole punched in the bottom. The PCR tube was placed in 1.5 ml microcentrifuge tube and the samples were centrifuged to collect and pellet the bone marrow cells. Cells were counted and resuspended in sterile PBS. For head-shielded chimeras, female CD45.2+ C57BL/6-Tyrc-2 mice were initially anaesthetized with ketamine–xylazine. Mice were placed into the irradiator with their head positioned under a custom-made, 5 mm thick lead frame. Three mice were fitted into this field and received a single dose of 9 Gy extracranial radiation. Mice were left to recover from anaesthesia and returned to their cages. Mice fed with sulfatrim-enriched diet for the first 3 months after irradiation. Within 24 h, mice were retro-orbitally infused with approximately 2 × 107 bone marrow cells from multiple pooled congenic CD45.1+ female donors (JAX, 002014), isolated as described above. Mice were left to recover after the transplant for 4 months before further experimentation. The recombination efficiency (CD45.1-to-CD45.2 ratio) was quantified at the end point in cells isolated from femoral and cranial bone marrow as described previously79, and in heparinized blood collected by cardiac puncture. Leptomeningeal cells were used for immunophenotyping with flow cytometry to assess the cranial and extracranial origin of selected immune cell types.
Immune cell depletions
cDC progenitors were depleted in bone marrow chimeras that received WT or Zbtb46-DTR+/+ donor cells with DTx (Sigma-Aldrich, D0564), diluted in PBS and delivered intraperitoneally. The initial dose of 400 ng DTx was injected 1 day before cancer cell implantation, followed by bi-weekly injection of 100 ng. Both WT and Zbtb46-DTR+/+ cohorts were receiving DTx. In experiments with only Zbtb46-DTR+/+ chimeras, approximately half of the mice received PBS as a vehicle. cDC progenitors were depleted in Clec9acreRosa26lsl-DTR mice, Clec9acre littermates (DTR genotype negative) were used as controls. DTx dosing was the same as for Zbtb46-DTR chimeras. NK cells were depleted with polyclonal rabbit anti-mouse asialo GM1 (Poly21460; BioLegend, 146002), rabbit polyclonal IgG was used as control (Invitrogen, 02-610-2). Both antibodies were reconstituted with PBS. An initial dose of 50 μg was instilled 1 day before cancer cell implantation, followed by bi-weekly injections of 50 μg until the end point (see the schematic in Fig. 5i). T cells were inactivated and depleted with anti-mouse CD3ε (145-2C11, BE0001, BioXCell), Armenian hamster IgG was used as control (BE0091, BioXCell). Anti-CD3ε antibodies were diluted in pH 7.0 buffer (IP0070, BioXCell). Isotype was co-diluted with rabbit polyclonal IgG for the experiment with parallel depletion of NK cells and T cells (Extended Data Fig. 4). An initial dose of 100 μg was instilled 1 day before cancer cell implantation, followed by tri-weekly injections of 100 μg until the end point.
Flow cytometry
Single-cell suspensions were prepared as described above and previously6. After filtering through a 70-μm filter and washing with 2 mM EDTA and 1% BSA in PBS, non-specific binding sites were blocked with TruStain FcX (BioLegend, 101320) diluted in PBS, supplemented with 10% rat serum (Sigma-Aldrich, R9759) for 10 min on ice. Antibodies against surface antigens were diluted in reconstituted Brilliant Stain Buffer Plus (BD, 566385), supplemented with 5% rat serum. Surface antigens were stained for 15 min on ice, except for panels that included CCR7 staining and were stained for 20 min at ambient temperature. LIVE/DEAD Green/Violet/FarRed Dead Cell Stain kits (Life Technologies, L34969, L34963, L34973, respectively), DAPI (Molecular Probes, D1306) or propidium iodide (Thermo Fisher Scientific, P3566) were used as viability stains. Buffer without BSA was used before LIVE/DEAD staining, which was performed for 15 min on ice. Red blood cells were lysed with 1× ACK buffer or 1× eBioscience RBC lysis buffer (Invitrogen, 00-4300-54) for 5 min at ambient temperature.
For cytokine production analysis, leptomeningeal isolates were resuspended in serum-free IMDM and incubated (MSKCC Media Core) with or without addition of brefeldin A (BioLegend, 420601), ionomycin (StemCell Technologies, 73722) and phorbol 12-myristate 13-acetate (PMA; Invivogen, tlrl-pma), for 2 h at 37 °C. Where the intracellular staining was performed, cells were further fixed with IC fixation buffer for 20 min (Invitrogen, 00-8222-49) at room temperature, permeabilized and stained with antibodies against intracellular markers in 1× permeabilization buffer for 1 h (Invitrogen, 00-8333-56) and analysed. For pSTAT1 transcription factor staining, cells were processed using True-Nuclear Buffer Set (BioLegend, 424401) or FOXP3 Fix/Perm buffer set (BioLegend, 421403) and analysed. MHC class I levels of vehicle- or recombinant mouse IFNγ-exposed (BioLegend, 714006) cells in vitro was measured 24 h after treatment. Data were recorded using LSR Fortessa (BD). Gating and analysis were performed essentially as described previously6,80, using unstained samples, isotype-stained samples and/or FMO controls (Supplementary Fig. 6). During the analysis of ex vivo profilings, B cells were assigned as B220+, T cells as CD3+, monocytes-macrophages as CD11b and Ly6C+ or F4/80+, neutrophils as Ly6G+, NK cells as Nk1.1+, conventional DCs as CD11c+MHC-II+ (B220−) and pDCs as CD11c+MHC-II+B220+. The cDC1 population was gated as XCR1+ cDCs, and the cDC2 population was gated as CD11b+ cDCs. Typically, percentages of all leukocytes (CD45+) assigned with high confidence are plotted. A list of the antibodies used for flow cytometry is provided in Supplementary Table 6.
Mature CCR7+ DC lineage tracing
For cell surface protein mapping, we used C57BL/6-Tyrc-2 mice. For genetic lineage tracing, we used the Xcr1cre-mCherry mouse strain (JAX, 035435) crossed with Rosa26lsl-eYFP (JAX, 007903). The expression of XCR1-driven mCherry was hemizygous. Cell suspensions from leptomeninges and spleen were stained with a DC-focused antibody panel (CD45 BUV395, CD11c BUV737, MHC-II IA/IE PerCP, XCR1 BV650, CD11b PE/Cy7, CCR7 APC, B202 AlexaFluor 700; Supplementary Table 6) and dead cells were marked with DAPI. Live, single-cell CD45+ leukocytes were selected and pDCs were excluded based on B220 expression, conventional DCs were then gated as CD11c+MHC-II+ cells. These were further divided into XCR1+ cDC1 and CD11b+ cDC2 subtypes. Expression of CCR7+ was established based on the FMO control. XCR1 and CD11b cell surface protein expression in cDCs is typically mutually exclusive. Mature CCR7+ DCs of cDC1 origin therefore co-expressed CCR7 and XCR1, whereas mature CCR7+ DCs of cDC2 origin co-expressed CCR7 and CD11b. XCR1+ cDC1 cells, both immature and mature, expressed mCherry and mCherry expression patterns reflecting the antibody staining. Mature CCR7+ DCs of cDC1 origin in this experiment therefore co-expressed XCR1, mCherry and CCR7. We included a chronic systemic inflammation control by intraperitoneal LPS delivery for 2 weeks (6 μg dose in saline, three times a week, E. coli O55:B5 lipopolysaccharide, Sigma-Aldrich, L2880)6. Staining and analysis was performed as described above.
Soluble protein detection in plasma and CSF
Solute analytes in the human and mouse CSF were analysed using following multiplexed bead arrays, used as recommended by the manufacturer: LEGENDPlex mouse anti-virus response (BioLegend, 740622), LEGENDPlex human CD8/NK panel (BioLegend, 740267). IFNγ levels in human CSF were measured with human IFNγ Quantikine HS ELISA Kit (R&D, HSDIF0).
NK cell in vitro survival assay
NK cells were enriched from dissociated spleens of female and male C57BL/6 mice with MojoSort mouse NK cell isolation kit (BioLegend, 480049). Approximately 20,000 cells were seeded into a 1:1 mixture of HG DME and human CSF from patients with cancer without or with LM containing 10 ng ml−1 recombinant human IL-2 (BioLegend, 589102), into a 96-well plate. Cells were incubated for 24 h with or without the addition of 1 ng ml−1 or recombinant mouse IL-12p70 (BioLegend, 577002) and recombinant mouse IL-15 (PeproTech, 210-15-10ug). Viability and cell counts were assessed with cytometry.
NK cell in vivo proliferation
Selected mice with injected with a dose of 100 μg 5-ethynyl-2′-deoxyuridine (EdU) in 100 μl saline intraperitoneally 4 h before transcardial perfusion with saline. Leptomeningeal cells were then collected and processed for flow cytometry. Surface staining was performed with polymer dye-tagged antibodies (CD45 BUV396 and Nk1.1 BV650; Supplementary Table 6), cells were stained for viability with LIVE/DEAD FarRed dye and further processed as described above. Cells were fixed with 1% MeOH-free paraformaldehyde in PBS and EdU click-it reaction was performed with Click-iT EdU AlexaFluor488 Flow Cytometry Assay Kit as recommended (C10632, Thermo Fisher Scientific).
Histology
Tissue from euthanized mice was fixed in 10% formalin overnight, thoroughly washed in tap water, sliced and stored in 70% ethanol until being embedded in paraffin. Paraffin-embedded blocks were then cut into 5-μm-thick sections and placed onto microscopy slides. H&E staining was performed by the MSKCC Molecular Cytology Core. Myelin staining was performed using the Luxol Fast Blue stain kit (Abcam, ab150675). Immunofluorescence was performed as described previously6, using following primary antibodies: CD11c (hamster, 1:50, Novus, NBP1-06651 and NB110-97871, used in combination), cleaved caspase 3 (rabbit, 1:200, Cell Signaling Technology, 9661S, CNPase (mouse, 1:1000, Abcam, ab6319), DCX (sheep, 1:200, R&D, AF10025), GFAP (goat, 1:500, Abcam, ab53554), IBA1 (rabbit, 1:500, Invitrogen, PA5-27436; and goat, 1:500, Novus, NB100-1028), MBP (mouse, 1:100, R&D, MAB42282), NeuN (mouse, 1:100–1:500, Sigma-Aldrich, MAB377) and OLIG2 (goat, 1:200, R&D, AF2418). AF488-, Cy3- and AF647-conjugated, anti-mouse, goat, rabbit and sheep secondary antibodies were obtained from Jackson ImmunoResearch; AF647-conjugated anti-hamster secondary antibodies were obtained from Abcam. For antibodies of murine origin applied on mouse tissue, the endogenous IgG was first blocked with reconstituted VisUBlock Mouse (R&D, VB001-01ML). DAPI (Molecular Probes, D1306) was used as nuclear counterstain. Autofluorescence was quenched with Vector TrueView (Vector Laboratories, sp-8400). Slides were scanned with a Mirax slide scanner (Zeiss), and images for further analysis were exported with CaseViewer (3DHISTECH).
Quantification of immunofluorescence imaging
Quantification of IBA1+ myeloid cells in the choroid plexus and IBA1+ microglia in cortical layers 3 and 4 was performed essentially as described previously19. Cleaved-caspase-3-positive cells in cancer plaques and clusters in leptomeninges were counted manually in fields of view of approximately equal size. The thickness of the periventricular GFAP+ layer was measured in both hemispheres and averaged per mouse. For MBP, the area of MBP+ tracts in both hemispheres was quantified and averaged per mouse. The relative fluorescence of DCX progenitors in the hippocampus was analysed in the corresponding AlexaFluor647 channel and reported as RFU. Cancer plaques in timepoint-matched AVV5-Ifng animals are rare; 2–3 fields of view per brain were extracted and the exact animal sample size and number of fields of view are stated in the corresponding images. Analysis of NeuN was done in the motor and somatosensory cortex in two regions. Region 1 covers layers 1–4 and region 2 covers layer 5 and 6. One section per animal was analysed and the chosen sections were between levels −0.18 mm to −0.196 mm relative to bregma. OLIG2 was quantified in the corpus callosum, spanning a lateral area from 0–1.7 mm relative to bregma; and together with CNPase in also in subcortical and cortical region above the corpus callosum. All image analyses were performed in Fiji/ImageJ81.
Statistical analysis and reproducibility
Plotting and statistical analysis were performed using Prism v.8.1.0 (GraphPad Software). Typically, experiments performed in vivo and ex vivo were compared using Mann–Whitney U-tests, unless specified otherwise. Metastasis incidences in the Ifngr1-proficient and -deficient mice were analysed using a χ2 test (Extended Data Fig. 6). Phenotypic changes in the in vitro cultured cancer cells after exposure to IFNγ were analysed using a t-test (Extended Data Figs. 7 and 8). Flow cytometry profiling of immune cell proportions across various conditions was analysed with multiple Mann–Whitney t-tests with Holm–Šídák correction. In the box plots (box and whisker plots), the box limits extend from the 25th to 75th percentile and the whiskers show minimum to maximum values. The results from single-cell analyses were plotted in Python. Bulk RNA-seq data were processed in R Studio. Mouse experiments are typically from at least two independent repetitions, except for Figs. 3g and 4a and Extended Data Figs. 3a,b, 4f–i, 5c,d, 7g,h, 8j, 11a,j and 14c–f; these in vivo experiments were performed once. Whenever possible, mice were randomly allocated into treatment groups. This was not possible in experiments with transgenic animals. Investigators were not blinded to the genotype or treatment over the course of experiment. Sample size and exact P values are included in the figures. Sample sizes were not predetermined. Critical mouse experiments (Figs. 2 and 3 and associated data) were reproduced by two independent investigators. Animal exclusion criteria for animal experiments (death within 3 days of injection or appearance of pigmented spots that interfered with BLI) are described above. One human CSF sample was excluded from IFNγ ELISA analysis as clinical chart review revealed the presence of sterile meningitis in this individual at the time of CSF collection.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.