Antibodies
All antibodies were purchased from Thermo Fisher Scientific unless specified otherwise. Primary antibodies for western blotting were used at a 1:4,000 dilution and secondary antibodies at 1:10,000 dilution. The following primary antibodies were used for western blotting: rabbit anti-TTYH2 (PA5-34395) raised against a 14-amino-acid N-terminal peptide; rabbit anti-APOE (16H22L18); rabbit anti-EEA1 (F.43.1); rabbit anti-LAMP1 (107); rabbit anti-RAB11 (20229-1-AP, Proteintech); rabbit anti-Na,K-ATPase (ST0533); rabbit anti-SERCA (JM10-20); rabbit anti-RAB7 (PA5-23138); and rabbit anti-GM130 (ARC0589). A goat anti-rabbit HRP-conjugated antibody (31460) was used as the secondary antibody for western blotting. The primary antibody used for immunocytochemistry was a rabbit anti-human-TTYH2 antibody (1:500 dilution; Antibodies-online.com) raised against the 455–534-amino-acid C-terminal peptide and a mouse anti-human-RAB9 (1:500 dilution; Mab9). The secondary antibodies used for immunohistochemistry was a goat anti-rabbit Alexa 488-conjugated antibody (1:200 dilution, A-11008) and goat anti-mouse Alexa 594-conjugated antibody (1:200 dilution, A-11032). The recognition of TTYH2 was confirmed for both anti-TTYH2 primary antibodies on purified protein (Supplementary Fig. 1a,b).
Mammalian and bacterial cell culture and strains
Suspension HEK293 GNTI− cells were grown in HyCell TransFx-H (Cytiva) medium supplemented with 1% FBS, 4 mM l-glutamine, 0.4% Poloxamer 188 and 100 U ml−1 penicillin–streptomycin at 37 °C and 5% CO2 while shaking. Proteins were expressed for 60 h unless specified otherwise. Adherent neuroblastoma cells (N2A, American Type Culture Collection) were grown in 10 cm dishes in Eagle’s minimum essential medium (Merck) supplemented with 10% FBS, 2 mM l-glutamine, 100 U ml−1 penicillin–streptomycin, 1 mM sodium pyruvate and nonessential amino acids at 37 °C and 5% CO2. Adherent HEK293T cells were grown in DMEM (Gibco) supplemented with 10% FBS and 100 U ml−1 penicillin–streptomycin at 37 °C and 5% CO2. N2A adherent cells were grown to full confluency before transfer to 245 × 245 mm dishes (Corning). The cells were further grown on these dishes for 2–3 days to 70–90% confluency and used for subcellular fractionation. The HEK293 TMEM16F knockout cells (provided by H. Yang) used for the cellular scrambling assays were grown in DMEM (Gibco) supplemented with 10% FBS and 100 U ml−1 penicillin–streptomycin at 37 °C and 5% CO2 in 10 cm dishes. All mammalian cell lines used in this study tested negative for mycoplasma infection.
For DNA preparation and protein expression, bacterial cells were grown in Terrific broth supplemented with 0.6% (v/v) glycerol and selection antibiotics with shaking. MC1061 chemically competent cells were used for the expression of sybodies. Chemically competent BL21 (DE3) cells were used for APOE expression. All DNA preparations were done in MC1061 cells.
Construct expression and purification
All cloning steps were carried out in suitably modified vectors using FX cloning43 or QuickChange mutagenesis (Stratagene, Agilent). Genes encoding human TTYH2 and TTYH3 (Genscript) were cloned into a pcDXC3MS vector (Addgene, 49030, suitable for protein expression in mammalian cells) and used for transient transfection of suspension HEK293 GNTI− cells. The TTYH2 in the pcDXC3MS construct was used as a template to generate the TTYH2(G165P/D166E/Q169R/F173R) mutant construct through site-directed mutagenesis44. The cells were transfected at a density of 1.5–2 × 106 cells per ml with PEI MAX, and valproic acid (4 mM) was added to stop cell division. After 60 h of expression, cells were collected and washed in PBS. Cell pellets were flash-frozen in liquid nitrogen and stored at −80 °C. On the day of purification, pellets were thawed and resuspended in lysis buffer (20 mM HEPES, pH 7.4, 200 mM NaCl, 2% (w/w) GDN, DNAse and protease inhibitors) at a ratio of 10 ml lysis buffer per 10 ml cell pellet. The resuspended cells were lysed for 1–2 h at 4 °C while rotating. The lysate was centrifuged at 15,000g for 30 min at 4 °C. The filtered (pore size of 5 µm) supernatant was added to 2 ml of Strep-Tactin resin per 50 ml cell pellet pre-equilibrated with SEC buffer (10 mM HEPES, pH 7.4, 200 mM NaCl and 50 µM GDN) and incubated in batches for 1 h at 4 °C while rotating. The flow-through was discarded and the resin was washed with 50 column volumes (CV) of SEC buffer. The protein was eluted with 5 CV of SEC buffer supplemented with 5 mM desthiobiotin, concentrated and loaded on a Superose 6 10/300 GL column for size-exclusion chromatography (SEC) in SEC buffer. Fractions corresponding to the protein peak were pooled, concentrated and used either fresh for the preparation of cryo-EM grids and reconstitution into liposomes or flash-frozen in liquid nitrogen with the addition of 10% glycerol and stored at −80 °C for later use in sybody-binding or APOE-displacement assays.
TTYH2 purified for sybody selection was concentrated after elution from Strep-Tactin resin and chemically biotinylated using EZ-link NHS-PEG4 biotin. Biotin was added at a 10-fold molar excess over TTYH2 monomer and the mixture was incubated for 1 h at 4 °C. Subsequently, Tris pH 7.4 was added to reach a concentration of 5 mM to quench the reaction and the tag was cleaved by the addition of 3C protease and incubation of the mixture on ice overnight. The next morning, biotinylated and cleaved TTYH2 was purified by SEC on a Superose 6 10/300 GL column to separate free biotin and the 3C protease. The peak fractions were pooled and concentrated, flash-frozen in liquid nitrogen after the addition of 10% glycerol and stored at −80 °C until use for sybody selection. Human ferroportin, used as a negative control in ELISA assays during sybody selections, was expressed, purified and biotinylated in the same way, except that the lysis buffer contained 2% and all other buffers with 0.04% (w/w) DDM instead of GDN.
The gene encoding human APOE3 including its signal peptide (Genscript) was cloned into a custom pcDX vector for mammalian expression fusing a 3C cleavage site and a His6 tag on the C terminus. This construct was used for the coexpression of both APOE and TTYH2 in HEK293 GNTI− cells. The cells were transfected with plasmids containing APOE and TTYH2 mixed in 1.1:1 molar ratio at a cell density of 1.5–2 × 106 cells per ml with PEI MAX. Valproic acid (4 mM) was added to stop cell division. A typical preparation for the coexpression of both proteins was carried out from a 0.6–0.9 l culture of transfected cells. After 38 h of expression, cells were collected and washed in PBS. Fresh cell pellets were used for the purification of the TTYH2–APOE complex. Cell lysis was performed as described above and cleared lysate was manually loaded onto a 1 ml bed of Strep-Tactin resin. The resin was washed with 15 ml SEC buffer and the protein complex was eluted in 5 ml SEC buffer supplemented with 5 mM desthiobiotin. The eluted complex was concentrated to 200 µl and separated on a Superose 6 Increase 5/150 GL column sequentially through the injection of 50 µl aliquots per run. The small column size was selected to shorten the elution time and to reduce the dissociation of the TTYH2–APOE complex. The peak fractions were concentrated to 1 mg ml–1 and used for the freezing of cryo-EM grids.
For its expression in E. coli, APOE3 was subcloned into p7XNH3 vector (Addgene, 47064) with the signal peptide (residues 1–18) removed. For lipid-transfer experiments in liposomes containing DGS-NTA(Ni) lipid, APOE3 in the p7XNH3 construct was modified by introducing a GSGSGSGSG linker between the 3C recognition site and the APOE3 gene. APOE3 was expressed in 3 l of BL21 cells for 3.5 h after induction. The cells were collected and cell pellets were flash-frozen in liquid nitrogen and stored at −80 °C. APOE was purified in its delipidated state using the strategy described for MSP purification45 but with minor adjustments. Frozen pellets were thawed and resuspended in 50 ml lysis buffer (50 mM Tris, pH 8, 500 mM NaCl, 1% Triton X-100, DNAse and protease inhibitors). The resuspended cells were lysed with a HPL6 high-pressure homogenizer (Maximator) and centrifuged at 15,000g for 30 min at 4 °C. The cleared lysate was loaded on 4 ml Ni-NTA bed resin pre-equilibrated in buffer containing 50 mM Tris, pH 8, 500 mM NaCl and 1% Triton X-100 by batch binding under rotation for 1 h at 4 °C. The flow-through was discarded and the resin was first washed with 50 ml of the equilibration buffer, followed by subsequent washing steps with 50 ml equilibration buffer containing 50 mM sodium cholate instead of Triton X-100 and with 50 ml equilibration buffer without detergent supplemented with 30 mM imidazole. The protein was eluted in 15 ml buffer containing 50 mM Tris, pH 8, 500 mM NaCl and 500 mM imidazole and the His10-tag was cleaved by the addition of 3C protease and incubation for 15 min at 4 °C. The cleaved protein was concentrated and loaded on a Superose 6 10/300 GL column equilibrated in 10 mM HEPES, pH 7.4 and 200 mM NaCl buffer. The peak fractions corresponding to the tetrameric delipidated APOE were pooled, concentrated and used for reconstitution into lipoprotein particles or flash-frozen in liquid nitrogen and stored at −80 °C to be used in displacement assays or for cryo-EM preparations. N-terminal (containing residues 19–209) and C-terminal (containing residues 191–317) fragments of APOE were subcloned into p7XNH3 vector, expressed and purified in the same way as described for the full-length construct.
For labelling with nanogold, APOE3 was purified as described above but without cleaving the His-tag. Next, 1.8 nm Ni-NTA-Nanogold (Nanoprobes) was mixed with APOE at a nanogold to APOE molar ratio of 1:5 and incubated for 15 min at room temperature. A low nanogold concentration was chosen to reduce nonspecific binding. Unbound nanogold was separated from the APOE–nanogold complex using a Sephadex G50 column. The nanogold-labelled APOE was mixed with purified TTYH2, TTYH3 or the TTYH2(G165P/D166E/Q169R/F173R) mutant at a 1:1 molar ratio and incubated on ice for 30 min. The complex was purified by SEC using a Superose 6 Increase 5/150 GL column. Peak fractions corresponding to the respective TTYH dimer were pooled, concentrated and used for the preparation of cryo-EM grids.
For lipid-transfer experiments in liposomes containing DGS-NTA(Ni) lipid, APOE was expressed in the modified p7XNH3 vector with the extended linker between the 3C recognition site and the APOE3 gene. The protein was purified as described above but without cleaving the His-tag. Sybodies were purified as previously described25. In brief, sybody constructs in a pSbinit (Addgene, 110100) vector containing a pelB signal sequence for periplasmic expression attached to their N terminus were expressed in 1 l of MC1061 E. coli culture for 14 h. The cells were collected and flash-frozen or used directly for purification. Cell pellets were resuspended in 50 ml TBS and lysed using a HPL6 high-pressure homogenizer (Maximator). The lysate was centrifuged at 15,000g for 30 min at 4 °C. The cleared lysate was used for batch binding on Ni-NTA resin with 4 ml bed volume pre-equilibrated in TBS under rotation for 1 h at 4 °C. The flow-through was discarded and the resin was washed with 50 ml TBS containing 30 mM imidazole. Sybodies were eluted in 15 ml TBS containing 500 mM imidazole, concentrated and purified by SEC on a Superdex 200 10/300 GL column. The peak fractions were pooled and concentrated to final protein concentration of 0.5–1 mM, flash-frozen and stored at −80 °C. For the isolation of endogenous TTYH2 and for the purification of TTYH2-containing cell-derived vesicles, Syb1 cloned in a pSbinit vector construct was modified for purification on Strep-Tactin resin. A Strep-Tactin-binding protein sequence was added at the C terminus following the His-tag sequence. Syb2 was subcloned into the pcDXC3VMS vector for mammalian expression containing a Venus fluorescent tag sequence on the C terminus following the 3C cleavage site. The 3C cleavage site was deleted for an uncleavable fusion of the sybody to Venus. The sybody was expressed in HEK293 GNTI− cells for 60 h. The cells were collected, washed in PBS and cell pellets were either flash-frozen in liquid nitrogen and stored at −80 °C or used directly for purification. The sybody was purified as described above.
Selection of synthetic nanobodies against TTYH2
TTYH2 in a pcDXC3MS vector was expressed in 3 l HEK293 GNTI− cells, purified and biotinylated as described above. The selection was carried out using mRNA libraries and vectors provided by M. Seeger as previously described25. In brief, chemically biotinylated TTYH2 (with 50% efficiency) was used in one round of ribosome display with concave, loop and convex synthetic libraries encoding synthetic nanobodies (termed sybodies), which primarily differ in the length of the CDR3 region. Each library contained around 1012 binders at the onset of the selection. The ribosome display output from the three libraries containing the DNA of captured sybodies was recloned into a vector for phage production and used for two rounds of phage display. The phage display output containing DNA of captured sybodies was subsequently subcloned into a pSbinit vector for sybody expression, and an initial pool of selected binders was identified by ELISA with TTYH2 as the target protein and ferroportin as the negative control. Clones with the highest signal over background were sequenced. Sybodies that showed promising biochemical properties were tested for their binding to TTYH2 by SEC (Supplementary Fig. 1b–d). TTYH2 supplemented with a 1.6 molar excess of the respective sybody was loaded on a Superose 6 Increase 5/150 GL column and the presence of the sybody in fractions containing TTYH2 was detected by SDS–PAGE. In this way, it was possible to isolate TTYH2 binders from all three libraries, two of which were used in this study, namely Sb1 from the concave library (with a short CDR3) and Sb2 from the loop library (with a medium CDR3).
Isolation of endogenous TTYH2 complexes
To isolate endogenously expressed TTYH2 in complex with potential interaction partners, 1.8 l HEK293 GNTI− cells at a density of 4 × 106 cells per ml was used. The cells were collected, washed in PBS and resuspended in 80 ml lysis buffer (20 mM HEPES pH 7.4, 200 mM NaCl and 2% (w/w) GDN). The lysate was incubated at 4 °C under rotation for 1 h. Next, 2.2 mg Sb1 was immobilized on Strep-Tactin resin with 0.5 ml bed volume by batch binding for 1 h at 4 °C under rotation. The resin was drained and the excess sybody was removed with 5 ml SEC buffer. The cell lysate was centrifuged at 15,000g for 30 min at 4 °C and manually loaded onto the resin containing immobilized Sb1. The resin was washed with 50 ml SEC buffer. The sybody-bound TTYH2 complexes were eluted with 2.5 ml SEC buffer supplemented with 5 mM desthiobiotin. Eluted complexes were concentrated using a centrifugal filter with 3 kDa MW cut-off and analysed by liquid chromatography coupled to mass spectrometry for identification of potential interaction partners (carried out by the Functional Genomics Center Zurich).
Subcellular fractionation
To investigate the cellular localization of TTYH2, we fractionated subcellular compartments by density centrifugation on a step sucrose gradient. To this end, 600 ml HEK293 GNTI− cells at a density of 2 × 106 cells per ml or neuroblastoma cells grown on two 245 × 245 cm plates to confluency were used per experiment. The cells were collected, washed in PBS and resuspended at a 1:1 volume ratio in buffer containing 8.25% (w/w) sucrose, 10 mM HEPES pH 7.4, 1.5 mM MgCl2, 30 µM cycloheximide and protease inhibitors. The cytoplasmic content was released by dounce homogenization with 15 strokes on ice. The homogenized cells were centrifuged twice at 2,000g for 10 min at 4 °C to separate the nuclear fraction. The post-nuclear supernatant (PNS) was applied on top of a step sucrose gradient. The gradient was created by layering 1 ml aliquots of buffer (10 mM HEPES pH 7.4, 1.5 mM MgCl2 and 30 µM cycloheximide) with decreasing sucrose content as follows: 35%, 25%, 20%, PNS (8.25%) for HEK293 cells and 25%, 20%, 15%, PNS (8.25%) for N2A cells. It was necessary to introduce a step with 15% sucrose for N2A cells to better separate the plasma membrane fraction. The sample was centrifuged at 210,000g for 3.5 h at 4 °C using 4.2 ml tubes and a SW 60 Ti swinging-bucket rotor (Beckman). The fractions were collected and analysed by western blotting with antibodies against TTYH2, APOE and organelle markers (Supplementary Figs. 2–6).
Reconstitution of lipidated APOE
All lipids in this study were purchased from Avanti Polar Lipids. Lipids were used as a chloroform solution and prepared by evaporating chloroform under a nitrogen stream and washing with diethyl ether. Excess solvent was evaporated by desiccation for 1 h and the lipid film was resuspended to 10 mg ml–1 in buffer containing 20 mM HEPES, pH 7.4, 100 mM KCl and 2 mM CaCl2 by sonication. Lipids were flash-frozen and stored at −80 °C. For cryo-EM preparations with lipidated APOE, a lipid mix containing POPE, POPG, egg PC and cholesterol at a 3:1:1:0.5 w/w ratio was used. For APOE used in FRET-based lipid-transfer assays, the lipids consisted of soy polar extract (Avanti, 541602) with additional 5% (w/w) NBD-PE (tail labelled, Avanti, 810156P). For APOE used in immunocytochemistry experiments, the lipids consisted of soy polar extract with additional 15% (w/w) rhodamine–PE (head labelled, Avanti, 810150). For APOE–mCherry used in immunocytochemistry experiments, the lipids consisted of POPE, POPG, egg PC and cholesterol at a 3:1:1:0.5 w/w ratio. APOE was expressed in bacteria and purified as described above. Lipoproteins were prepared using the cholate dialysis method46. Lipids were solubilized in sodium cholate at a 1:1 molar ratio, and purified APOE concentrated to 4 mg ml−1 was mixed with solubilized lipids at a 1:100 APOE to lipid molar ratio in a reaction volume of 0.3–0.7 ml. The mix was incubated for 16 h at 4 °C under rotation. The detergent was removed by dialysis in buffer (added at 5,000× higher volume) containing 10 mM Tris, pH 8 and 150 mM NaCl in two steps over the course of 2 days. The final lipoprotein complexes were separated from aggregates and degradation products by SEC on a Superose 6 10/300 GL column equilibrated with 10 mM HEPES, pH 7.4 and 200 mM NaCl. Fractions containing intact lipidated APOE were pooled, concentrated, flash-frozen and stored at −80 °C.
APOE–mCherry purification and lipidation
The APOE3 gene not containing a signal peptide was subcloned into a custom pcDx vector for mammalian expression as an N-terminal mCherry fusion protein. The vector contained a streptavidin-binding protein tag, a MYC tag, the mCherry sequence and a 3C cleavage site. HEK293 GNTI− cells were transfected at a density of 1.2 × 106 per ml with PEI MAX, and valproic acid was added to stop cell division. After 60 h of expression, cells were collected and washed in PBS, and cell pellets were used directly for APOE–mCherry purification per the procedure described for APOE purification from E. coli cells. After purification by SEC, fractions containing APOE were pooled and concentrated. Lipidation was carried out using a lipid mix containing POPE, POPG, egg PC and cholesterol at a 3:1:1:0.5 w/w ratio with the cholate dialysis method as described above. After dialysis, lipidated APOE–mCherry was directly used for incubation with cells.
Immunocytochemistry and confocal microscopy
Adherent HEK293 cells were seeded in a 12-well plate with round cover slips placed inside each well to a confluency of 50–80%. The cells were washed in PBS and fixed by incubation in 4% paraformaldehyde in PBS for 15 min. The reaction was quenched by adding 10 mM glycine in PBS for 10 min. Cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min. The excess detergent was washed off and the cells were blocked in 2% BSA–PBS for 15 min. The cells were incubated with the first antibody diluted in 2% BSA–PBS for 2 h. Excess antibody was washed off and a secondary antibody diluted in 2% BSA–PBS was added and incubated for 1 h. The cells were washed with 2% BSA–PBS and the cover slips were mounted on microscope slides using Vectashield antifade mounting medium with DAPI (AdipoGen). For double-antibody staining, the cells were first stained with the anti-TTYH2 and the Alexa-488-conjugated anti-rabbit antibodies and then with the anti-RAB9 and the Alexa-594-conjugated anti-mouse antibodies.
For detection of colocalization of TTYH2 and APOE in HEK293 cells, in vitro lipidated APOE–mCherry was added at 0.9 µM to cells seeded in a 12-well plate and incubated for 20 min in an incubator at 37 °C and 5% CO2. After incubation, cells were fixed and stained with anti-TTYH2 and Alexa-488-conjugated anti-rabbit antibodies as described above. For detection of colocalization of endocytosed lipids and TTYH2 in HEK293 cells, APOE lipidated in vitro using a lipid mix containing 15% rhodamine–PE was added at 2 µM to cells seeded in a 12-well plate and incubated for 30 min in an incubator at 37 °C and 5% CO2. After the incubation, cells were fixed and stained with anti-TTYH2 and Alexa-488-conjugated anti-rabbit antibodies as described above. All samples were analysed using a Zeiss LSM 980 Airyscan inverted confocal laser scanning microscope at the Center for Microscopy and Image Analysis (ZMB) of the University of Zurich (UZH). z-stacks of images were acquired from multiple locations and processed in Fiji47.
Preparation of cell-derived vesicles containing TTYH2
For the preparation of cell-derived vesicles for structural studies, a construct of the human TTYH2 gene in a pcDXC3MS vector not containing a Strep-Tactin-binding protein sequence was expressed in HEK293 GNTI− cells for 60 h. A typical sample was obtained from 4 l of culture. Cells were initially collected and washed in PBS. Vesicles from total cell membranes were prepared as previously described48 but with minor adjustments. Cells were resuspended in 100 ml buffer containing 20 mM HEPES, pH 7.4, 300 mM KCl, 1 mM MgCl2, DNAse and protease inhibitors. The resuspended cells were dounce homogenized on ice with 30 strokes and then sonicated (on ice at 60% power with 4 × 30 s pulses interrupted by 30-s intervals). The sonicated lysate was centrifuged twice at 12,000g for 10 min at 4 °C. After the first spin, 5 mM EDTA was added to the supernatant to prevent vesicle aggregation. The supernatant was loaded onto Q Sepharose resin with 20 ml bed volume pre-equilibrated with 20 mM HEPES, pH 7.4, 300 mM KCl, 1 mM MgCl2 and 2 mM EDTA buffer to remove nucleic acids. The flow-through was collected and the resin was washed with 20 ml equilibration buffer. The wash and flow-through were pooled. Next, 2.5 mg Sb1 was immobilized on a 1.5 ml bed of Strep-Tactin resin by batch binding for 1 h at 4 °C while rotating. The excess sybody was washed away and the resin loaded with sybody was used to capture vesicles containing TTYH2 in the outside-out orientation. Pooled wash and flow-through fractions were mixed with the Sb1–Strep-Tactin resin and incubated for 1 h at 4 °C while rotating. Flow-through was discarded and the resin was washed with 100 ml of 20 mM HEPES, pH 7.4, 300 mM KCl and 2 mM EDTA buffer and vesicles were eluted with 15 ml wash buffer supplemented with 5 mM desthiobiotin. The vesicles were concentrated using a centrifugal filter with 100 kDa MW cut-off and used for the freezing of cryo-EM grids. For the sample containing lipidated APOE, the expression time was reduced to 40 h and vesicles were prepared as described above.
Sybody-displacement assay
As classical binding experiments such as microscale thermophoresis turned out to be unsuitable (Supplementary Fig. 7), we probed the site specificity of APOE binding to TTYH2 using Sb2, which occupies a similar epitope. Sb2 was expressed as a fusion with Venus fluorescent protein on its C terminus in HEK293 GNTI− cells and its displacement from the complex with TTYH2 was monitored by fluorescent SEC49. The Sb2–Venus construct was mixed with TTYH2 purified from 1.2 l HEK293 GNTI− cells at a 3:1 molar ratio and incubated on ice for 30 min. The complex was subsequently purified by SEC on a Superose 6 10/300 GL column. Peak fractions at an appropriate elution volume corresponding to the complex (detected by the measurement of the absorption 280 nm and confirmed by SDS–PAGE) were pooled, kept on ice overnight and used for displacement assays the next day. Sb2 displacement was analysed using unlipidated APOE, its N-terminal domain and lipidated APOE. Displacement with unlabelled Sb2 served as the positive control. Every reaction contained 40 µl of the TTYH2–Sb2–Venus complex and one of the three competitors at various concentrations. After 15 min of incubation on ice, each sample was injected onto a Superose 6 Increase 5/150 GL column equilibrated with SEC buffer and the fluorescence intensity of Sb2–Venus was recorded for 20 min. The displacement was quantified by the decrease in fluorescence of the complex and the concomitant increase in fluorescence of the free sybody fusion. The averaged values were compared with the displacement of Sb2–Venus by the unlabelled Sb2, which was assigned as 100%.
Reconstitution of TTYHs into 85% DPPC liposomes
For the lipid-transfer assays, TTYH2 was reconstituted into liposomes with an 85% (w/w) DPPC content to reduce their nonspecific interaction with APOE. A lipid mix containing 85% DPPC, 14% POPC and 1% rhodamine–PE (head labelled, Avanti, 810150) (w/w) was prepared as described above and the lipid film was solubilized in 20 mM HEPES pH 7.4, 100 mM KCl, 2 mM CaCl2 and 35 mM CHAPS by sonication at a lipid concentration of 10 mg ml−1. For the experiments with APOE–His10 tethering, the lipid mix contained 85% DPPC, 13.5% POPC, 1% rhodamine–PE and 0.5% DGS-NTA(Ni) (Avanti, 790404) (w/w). Lipids were flash-frozen and stored at −80 °C.
Before mixing with either purified TTYH2 or TTYH3, or buffer in case of mock liposomes, the lipids were diluted to 4 mg ml−1 in the same buffer. Constructs of TTYH2 and TTYH3 in a pcDXC3MS vector were expressed in HEK293 GNTI− cells and purified as described above. The purified protein was concentrated after SEC to 2–4 mg ml−1 and mixed with the CHAPS-solubilized lipids at a lipid to protein ratio of 50 (w/w). The mix was incubated for 15 min while rotating. To remove the detergent, 100 mg biobeads per 1 ml lipids was added and the mix was incubated for 30 min under rotation. Six additional aliquots of biobeads were added over the course of 3 days to ensure complete removal of the detergent. The entire reconstitution process was carried out at room temperature. Liposomes were collected by centrifugation at 200,000g for 30 min at 21 °C and resuspended to 10 mg ml−1 lipid in 20 mM HEPES pH 7.4, 100 mM KCl and 2 mM CaCl2 buffer. Liposomes were flash-frozen and stored at −80 °C. The reconstitution efficiency was tested by SDS–PAGE and amounted to 20–30% for both TTYH2 and TTYH3 liposomes. Protein integrity was confirmed by analytical SEC of samples re-extracted from liposomes in detergent GDN.
Lipid-transfer assay with fluorescent lipids
To detect lipid transfer between APOE and TTYH2, we used a liposome-based system using lipids containing complementary fluorophores that form a FRET pair. TTYH2 and TTYH3 were reconstituted into liposomes containing 1% rhodamine–PE, 85% DPPC and 14% POPC (w/w) as described above. APOE was lipidated in vitro using lipids from soy polar extract supplemented with 5% (w/w) NBD–PE as described above. The fluorescence of NBD was monitored using a Fluoromax Horiba spectrofluorometer with excitation at 460 nm, emission at 535 nm and a bandwidth of 5 nm. The signal was recorded every 0.1 s. A quartz cuvette was loaded with 2 ml liposomes extruded through a 400 nm membrane and diluted to 0.2 mg ml−1 with 20 mM HEPES, pH 7.4, 100 mM KCl and 2 mM CaCl2 buffer. After recording of a baseline for 50 s, lipidated APOE containing NBD–PE was added to 50 nM and NBD fluorescence was recorded for 250 s. After this time period, Triton X-100 was added to a concentration of 0.1% to completely solubilize liposomes and the NBD signal increase was recorded for another 100 s. The data were normalized using the following formula: (F – F10)/(F400 – F100), where F is the fluorescence intensity at every time point, F10 is the fluorescence intensity at 10 s and F400 is the fluorescence intensity at 400 s. To estimate the effect of APOE tethering on the observed lipid-transfer acceleration, we spiked the liposomes with the DGS-NTA(Ni) lipid and used APOE with a hexahistidine-tag, therefore forcing its binding to the liposome surface. The His-tagged APOE was lipidated in vitro using lipids from soy polar extract supplemented with 5% (w/w) NBD–PE as described above and was added to the liposomes containing 85% DPPC, 13.5% POPC, 1% rhodamine–PE and 0.5% DGS-NTA(Ni) lipids (w/w). NBD fluorescence was recorded and the data were analysed as described above.
Scrambling assays
To probe whether TTYH2 catalyses lipid movement between the two bilayer leaflets, we used a liposome-based assay and a cellular assay. For the liposome-based assay, TTYH2 was reconstituted into liposomes containing lipids from soy polar extract, 20% cholesterol and 0.5% NBD–PE (head-labelled, Avanti, 810145). The liposomes were prepared either by solubilizing lipids in buffer containing 35 mM CHAPS and used for TTYH2 reconstitution as described above or by resuspending lipids in detergent-free buffer. The latter lipid batch was used for liposome reconstitution of TTYH2 by gradually destabilizing liposomes with small amounts of Triton-X 100 as previously described1. In brief, the lipids were extruded using a 400 nm membrane and diluted to 4 mg ml−1 in the liposome buffer containing 20 mM HEPES pH 7.4, 100 mM KCl and 2 mM CaCl2. The liposomes were titrated with 10% Triton-X 100 and destabilization was monitored by measuring absorbance at 540 nm. TTYH2 purified in detergent was added to the destabilized liposomes at a lipid to protein ratio of 100 (w/w) and detergent was gradually removed using biobeads. The liposomes were collected by centrifugation at 200,000g and the liposome pellet was resuspended in the liposome buffer to 20 mg ml−1 lipid, aliquoted and flash-frozen in liquid nitrogen for storage at −80 °C. The reconstitution efficiency was tested by SDS–PAGE and amounted to 30–40% for both solubilized and destabilized preparations. Protein integrity was confirmed by analytical SEC of samples re-extracted from liposomes in detergent GDN (Supplementary Fig. 8).
The liposome-based scrambling assay was performed as previously described1. NBD fluorescence was monitored using a Fluoromax Horiba spectrofluorometer with excitation at 460 nm, emission at 535 nm and a bandwidth of 2 nm. The signal was recorded every 0.1 s. A quartz cuvette was loaded with 2 ml of liposomes extruded using 400 nm membrane and diluted to 0.2 mg ml−1 with the liposome buffer. After recording of a baseline for 50 s, freshly prepared sodium dithionite was added to 30 µM and NBD fluorescence was recorded for 350 s. The data were normalized using the formula: F/F50, where F50 is the fluorescence intensity at 50 s (Supplementary Fig. 8).
Cell-based scrambling assays50 were performed using a HEK293 TMEM16F knockout cell line. Cells grown to 80–90% confluency were added to a 96-well polylysine-coated plate at a seeding density of 10%. The seeded cells were transfected with 100 ng DNA per well using Lipofectamine 3000 (ThermoFisher). The cells were transfected with pcDXC3MSV plasmid containing TTYH2, TMEM16F or the TMEM16F(F518H) constitutively active mutant, or an empty vector. The constructs contained Venus as a C-terminal tag for detection of transfected cells. At 48 h after transfection, the medium was replaced with imaging buffer containing 10 mM HEPES, pH 7.4, 25 mM glucose, 2 mM glutamax, 1.5 mM sodium pyruvate, 140 mM NaCl, 2.5 mM CaCl2, 5% Annexin V Alexa Fluor 594 conjugate and 5 nM Sytox red. Data were acquired using a GE InCell analyzer 2500 HS microscope at ZMB, UZH as a time series at ×10 magnification in the green (Venus, transfection control), red (Annexin V, exposure of PS on the cell surface) and far red (Sytox, cell death) channels with images acquired every 10 s. Data were analysed in Fiji47. Cells displaying a Venus signal were selected as regions of interest and used for quantifying the fluorescence intensity in the Annexin V channel for all frames. Dead cells were excluded. The fluorescence intensity was quantified over multiple cells and normalized by the number of cells. The normalized values at 500 s after start of the recording were plotted.
Dynamic force spectroscopy
MLCT cantilevers (Bruker AFM Probes) were functionalized through a gas-phase protocol. For initial cleaning, cantilevers were immersed in 2 ml CHCl3 in a PTFE vessel for 5 min. The procedure was repeated three times. After immersion, cantilevers were dried under a nitrogen stream. For functionalization, each cantilever was placed on a piece of Parafilm inside a polystyrene Petri dish, which was placed inside a desiccator along with 3× 30 µl of (3-aminopropyl)-triethoxysilane (APTES) and 3× 10 µl triethylamine in separate polystyrene caps. The cantilevers were incubated in the sealed desiccator for 2 h, flushed with argon and sealed for an additional 2 days to cure the amino functionalization. For functionalization of cantilevers with an aldehyde linker and TTYH2, 3.3 mg aldehyde-Ph-PEG24-NHS linker (BroadPharm) was dissolved in 500 µl DMSO in a PTFE vessel. Next, 30 µl triethylamine was added and mixed. The cantilevers were immersed in this solution for 2 h and subsequently cleaned in 2 ml CHCl3, similar to the initial cleaning step. Cantilevers were then placed on a piece of Parafilm in a polystyrene Petri dish. Next, 100 µl TTYH2 (at a concentration of 3.3 µM) in SEC buffer and 2 µl of 1 M sodium cyanoborohydride stock solution (prepared by dissolving 13 mg NaCNBH3 in 20 µl of 100 mM NaOH and 180 µl H2O) was applied to each cantilever and incubated for 2 h followed by the addition of 5 µl of 1 M ethanolamine and a further 15 min of incubation. A final cleaning step involved immersing the cantilevers in SEC buffer, similar to the initial cleaning step. Cantilevers were stored in SEC buffer at 4 °C until use in measurements. The preparation of APOE surfaces followed a similar protocol to the preparation of the cantilevers, with specific modifications. As a linker, 1 mg acetal-PEG-NHS linker (Creative PEGWorks) in 0.5 ml DMSO was used. Following linker incubation, glass slides were immersed in 1% citric acid for 10 min and afterwards cleaned in H2O, similar to the initial cleaning step. After the citric acid wash, low-height measurement chambers were mounted. All subsequent steps were performed in these chambers. For immobilization, 2 µM APOE solution in SEC buffer was applied followed by a final cleaning step involving three times exchange of the buffer solution as described for the initial cleaning step. Slides with mounted chambers were filled with SEC buffer and stored in sealed Petri dishes at 4 °C until used in measurements. An equivalent protocol was used for immobilization of Sb2.
Dynamic force spectroscopy measurement protocol
Measurements were conducted in a sound-isolated and vibration-isolated chamber at room temperature using a JPK NanoWizard 4 atomic force microscope (Bruker) and JPK-SPM software (v.6.4.22). TTYH2–PEG-functionalized cantilevers were calibrated for spring constant in contact mode and thermal noise measurements51,52,53. Experiments were carried out in SEC buffer at neutral pH and at acidic pH in buffer containing 10 mM MES pH 5.5, 200 mM NaCl and 50 µM GDN using 3 different cantilevers and 3 APOE surfaces across 8 pulling velocities (0.1, 0.2, 0.5, 1, 2, 5, 10 and 20 µm s–1). For each velocity, 1,000 force-displacement measurements were performed on a 10 × 10 grid (1 × 1 µm), with 10 measurements per grid point. Cantilever-bending corrections and analyses were conducted using JPK-Data processing software (DP-v.6.4). Further analysis, including Bell–Evans fitting for koff and Xβ values, was performed using in-house software developed in Python. During the approach and subsequent retraction of the cantilever, stochastic binding events were observed for both immobilized APOE (at pH 7.4 and 5.5) and Sb1 (at pH 7.4), which allowed the determination of the unbinding force Fu (Fig. 2e). Fu is directly correlated with the dissociation kinetics of the complex under an applied force and therefore depends on the loading rate (that is, the rate of force increase before unbinding)27,28,29. By varying the retraction speed while keeping the contact time constant, statistical analyses of Fu as a function of the loading rate (defined as the product of retraction velocity and the effective spring constant of the cantilever, Fig. 2f) were performed. This resulted in a linear dependence of Fu on the logarithm of the loading rate, which can be interpreted as a linear decrease in the free energy for dissociation, consistent with expectations for a single sharp energy barrier along the dissociation path28 (Fig. 2f). Thus, the separation of the energy barrier from the equilibrium position Xβ and koff were determined30,31,32. For competition experiments, 1,000 force displacement measurements were performed in the presence of 20–50 µM soluble Sb2 in the measurement buffer, and the reduction in binding events was analysed.
Cryo-EM grid preparation and data collection
For the preparation of cryo-EM samples of TTYH2 in complex with sybodies, TTYH2 was concentrated to 2 mg ml−1 and the sybodies were added at a 1:6 molar ratio of TTYH2 dimer to sybody and the samples were incubated for 30 min on ice before application on grids. For the preparation of a cryo-EM sample of APOE labelled with nanogold in complex with TTYH2, TTYH3 or the TTYH2(G165P/D166E/Q169R/F173R) mutant, the TTYH–APOE complexes were subjected to SEC and concentrated to 0.4 mg ml−1. For the preparation of cryo-EM samples from the expression of both TTYH2 and APOE, the complex was concentrated to 0.7–1.5 mg ml−1. For the sample of TTYH2 in complex with unlipidated APOE, the proteins were purified separately. TTYH2 was concentrated to 1.4 mg ml−1 and mixed with APOE at 1:2.3 molar ratio. The sample was incubated for 30 min on ice before application on grids. The sample of TTYH3 containing delipidated APOE was prepared equivalently. For TTYH2 in complex with lipidated APOE, TTYH2 was concentrated to 1 mg ml−1 and mixed with lipidated APOE at a 1:2.5 molar ratio. The sample was applied on grids immediately after mixing. For TTYH2 in cell-derived vesicles, the sample was concentrated to A280 = 2.7. TTYH2 vesicles prepared for interaction studies were concentrated to A280 = 9.1 and mixed with lipidated APOE immediately before application on grids. The final concentration of APOE was 45 µM and the vesicles were diluted twice by the addition of APOE.
Holey carbon grids Au 200 mesh R1.2/1.3 (Quantifoil) were used for all samples except the cell-derived vesicles, which were frozen on Au 300 mesh R1.2/1.3 (Quantifoil). Grids were freshly glow-discharged for 30 s. A volume of 2.5 µl sample was applied per grid. The blotting times varied between 2 and 4 s. Grids were plunge-frozen in liquid ethane–propane mix using Vitrobot Mark IV (Thermo Fisher Scientific) set to 4 °C and 100 % humidity. After vitrification, grids were stored in liquid nitrogen. For samples with cell-derived vesicles, the Vitrobot was set to 20 °C and 100% humidity. A volume of 3.5 µl vesicles was applied and incubated on the grid for 1.5–2 min before the liquid was manually removed with filter paper. Subsequently, another 3.5 µl aliquot of vesicles was applied and incubated for 20 s, with excess liquid removed by blotting for 3 s using a Vitrobot before grids were plunge-frozen and stored in liquid nitrogen.
The grids were imaged on a Titan Krios G3i (Thermo Fisher Scientific) with a 100 µm objective aperture at the ZMB of UZH. All data were acquired using a post-column energy filter (Gatan) with a 20 eV slit and a K3 direct electron detector (Gatan) in super-resolution mode. All micrographs were recorded with a defocus range from −1 to −2.4 µm using EPU 2.9 + AFIS faster acquisition (Thermo Fisher Scientific) at a nominal magnification of ×130,000 corresponding to a pixel size of 0.651 Å pixel−1 (0.3255 Å pixel−1 in super-resolution) with a total exposure time of 1.26 s (47 individual frames). The total electron dose on the specimen level varied between 60 and 69 e− Å−2 for different datasets.
Cryo-EM data processing
All cryo-EM datasets were processed in cryoSPARC54, except for the datasets of detergent-purified TTYH2 in complex with lipidated APOE, which were processed in Relion55. A box size of 440 pixels was used for processing throughout unless specified otherwise. Datasets of TTYH2 expressed with APOE and TTYH2 in complex with delipidated APOE, as well as TTYH3 supplemented with delipidated APOE, were processed following a similar scheme. The micrographs were motion and CTF corrected and the particles were picked using the Template picker with the TTYH2 map obtained in our previous study1 for template generation. Initially picked particles were 2D classified and particles with TTYH2 features were used to generate an ab initio volume. The map was improved by homogenous and nonuniform refinement to high resolution (2.8–3.5 Å). At this stage, only density of TTYH2 but not of APOE was visible. To isolate the particles that contained the TTYH2–APOE complex, we used 3D variability analysis with a loose mask covering only the top of TTYH2, where the interaction with APOE was expected. This enabled the isolation of particle subsets that contained the TTYH2–APOE complex, which were used for training of the Topaz neural network for more precise particle picking. Particles picked using Topaz56 were analysed using the same pipeline starting from 2D classification. After a final 3D variability analysis, particles that contained the TTYH2–APOE complex were used to create an ab initio map, which was refined by homogenous and nonuniform refinement.
Datasets of TTYH2 in complex with sybodies were processed following the same strategy as described above. For this dataset, the sybody density was already observed in early-stage ab initio maps. However, 3D variability analysis was still necessary for filtering out misaligned particles and to improve the quality of the map.
The datasets with TTYH2 purified in detergent in complex with lipidated APOE were processed in Relion57. Motion-corrected and CTF-corrected micrographs were used for particle picking using the TTYH2 map as a 3D reference. Particles were extracted with a box size of 440 pixels with 4× binning, which turned out to be optimal for this case (Supplementary Fig. 9). After several rounds of 2D classification, a subset of particles with visible APOE density was used to generate an ab initio map. This map was refined and used as a reference for a 3D classification with 10 classes and a regularization parameter of T = 20 on a large subset of particles. Particles from promising classes were selected for further 3D classification with similar parameters. At each step, the maps for 3D references were generated by ab initio reconstruction and refinement. Final particle subsets were used for generating maps by ab initio reconstruction and refinement and the final maps were sharpened.
For the dataset of TTYH2, TTYH3 and the TTYH2(G165P/D166E/Q169R/F173R) mutant with nanogold-labelled APOE, we collected 707, 3,000 and 2,486 micrographs, respectively, which produced particle sets of sufficient size for obtaining low-resolution reconstructions. Motion-corrected and CTF-corrected micrographs were used for template particle picking using the TTYH2 or TTYH3 map for the generation of templates. Particles picked on carbon areas were excluded. The particles were 2D-classified to remove junk and the final set of particles was refined by homogeneous refinement using the TTYH2 or TTYH3 map as input volume.
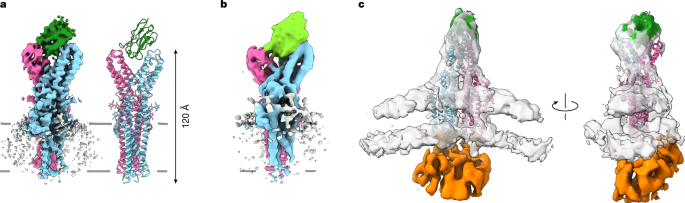
For processing of the datasets with TTYH2-containing cell-derived vesicles, the ‘re-center 2D classes’ option was switched off in all 2D classification jobs. Motion-corrected and CTF-corrected micrographs were used for manual particle picking. About 500 manually selected particles with side and top views of TTYH2 were used for initial Topaz-based particle picking. These particles were 2D classified, and the best classes were used for a second round of Topaz-based picking. The iterative process was repeated until a homogenous subset of particles was obtained with clear TTYH2 features visible on 2D class images. This subset was used for ab initio reconstruction. Initially, the particles were extracted in a comparatively small box size of 280 pixels, which was necessary to assist the alignment process. The ‘center structures in real space’ option was switched off in all ab initio jobs. The initial map was refined using the homogenous refinement option. The refined particles were re-extracted in a box size of 440 pixels and refined again in the new box size. The obtained map was locally refined focusing on the protein and excluding the membrane. The final map was sharpened.
TTYH2 in cell-derived vesicles in complex with lipidated APOE was processed in a similar manner as for the dataset containing TTYH2 vesicles alone. Particle picking was initially carried out manually and then interactively with Topaz. Particles were first extracted in a box size of 280 pixels. Particles from the best-looking 2D classes with TTYH2 features were used in an ab initio job with the ‘center structures in real space’ option switched on. When this option was switched off, as described for the previous dataset, the APOE density vanished as a result of averaging. The ab initio map was refined and the refined particles were used in a 3D classification job with the protein density masked. The 3D classification helped to separate the particle subsets with the APOE density bound to TTYH2. The new set of particles containing APOE was refined again and the refined particles were re-extracted in a box size of 440 pixels. The re-extracted particles were then used as input for homogenous refinement and the refined volume was sharpened.
Model building and refinement
The cryo-EM structure of TTYH2 in detergent (Protein Data Bank (PDB) accession 7P54) was used to build the TTYH2 models in complex with Sb1 or lipids. TTYH2 was initially placed into the cryo-EM density by rigid body fitting in Chimera58,59. The structure of the GFP-binding nanobody (PDB 3K1K) with variable regions removed was used as an initial scaffold for the modelling of Sb1. The nanobody was placed into the density in Chimera and the CDR loops were manually edited to match the Sb1 sequence in Coot. The structure of the complex was refined in Phenix60. For the high-resolution structure of TTYH3, the cryo-EM structure of TTYH3 in detergent (PDB 7P5C) was fitted into the cryo-EM density in Chimera and the structure was refined in Phenix. For the high-resolution structure of TTYH2 with bound lipids, TTYH2 was fitted into the cryo-EM density in Chimera and lipids were manually placed in Coot61. The structure was refined in Phenix. For all cryo-EM maps of TTYH2 in complex with APOE, the TTYH2 model was fitted into the density in Chimera. Figures containing molecular structures and densities were prepared using DINO (http://www.dino3d.org) and ChimeraX59.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.