Ethical compliance
All animal procedures performed in this study were approved by the UK Government (Home Office) and by the Crick Institutional Animal Welfare Ethical Review Panel.
Mice
Animals were housed in individually ventilated cages on a 12/12-h light–dark cycle (lights on: 22:00–10:00) at 21 °C and 32% humidity with food and water available ad libitum. Standard mouse chow (2018S Teklad Global 18% Protein Rodent Diet) was used in all experiments. Baseline (Pre) behavioural testing was performed in the first 4 h of the dark phase, and testing after food deprivation (Post) was performed 6 h after the start of the Pre phase, unless stated otherwise.
C57BL/6J mice (Mus musculus) from the Crick breeding colonies were used at age 8–14 weeks for all behavioural experiments. Agrp-cre mice32 (The Jackson Laboratory, JAX 012899) were used to target ArcAgRP neurons. For slice physiology experiments, this line was crossed to Cre-dependent Rosa26 Tomato mice (Ai9, The Jackson Laboratory, JAX 007909). For hormone receptor KO experiments, Esr1loxP (oestrogen receptor α conditional KO, imported from EMMA, EM:11179)60 or PrloxP (progesterone receptor conditional KO, made in-house)3 were used. All lines were maintained in a C57BL/6J background. Unless otherwise noted in the figure legends, all experiments were performed in female mice.
Behavioural profiling
Virgin females without previous pup exposure were used in all experiments. For experiments in pregnant females, virgin females were paired up with an experienced stud male until a vaginal plug was detected, which marked pregnancy day 1 (D1). Behavioural scoring and analysis were performed by an individual blind to the experimental condition of the animal (for example, Pre versus Post, manipulation versus control).
Pup-directed behaviour assay
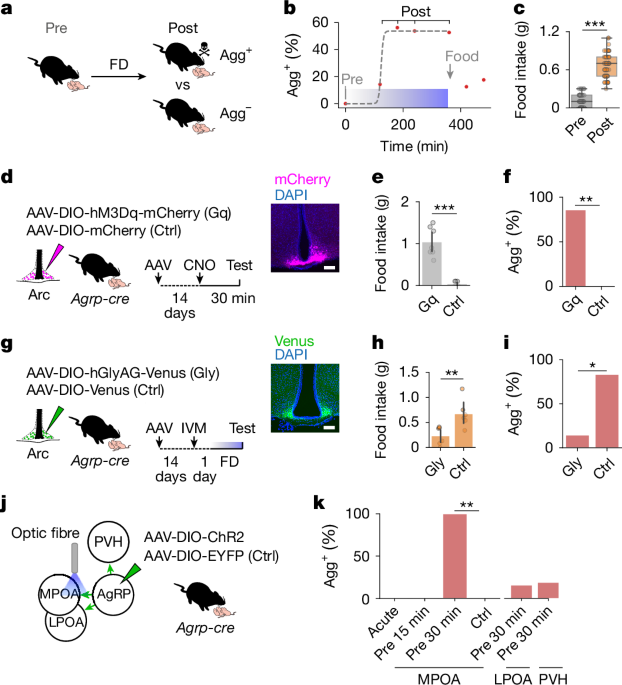
Animals were individually housed for 4 days before behavioural testing. Experiments were performed in the home cage and were preceded by a 10-min habituation period. Two C57BL/6J pups 1–3 days of age were placed in different corners opposite the nest, and pup interactions were recorded for 15 min with a Basler Ace GigE, acA1300-60gmNIR camera. Videos were acquired at a frame rate of 30 Hz using a custom protocol written in Bonsai (NeuroGEARS, https://bonsai-rx.org/) and behaviours were scored using behavioural observation research interactive software (BORIS)61. Pup-directed behaviours were classified as follows: contact latency was defined as the time elapsed until the first contact of the test animal’s nose with a pup; pup grooming was defined as physical contact with pups involving licking, pup displacement and rhythmic head movements; and pup chemoinvestigation was defined as close interaction with the nose of the animal touching the pup but no additional physical contact. The onset of pup retrieval was defined by the time elapsed until a pup was picked up and retrieved to a nest. Time in nest was defined as the time the female mouse stayed in the nest with at least one pup. Crouching was defined as the female mouse stationarily positioned over pups in the nest. Total parenting time was calculated as the sum of time spent grooming pups, retrieving pups and time spent in the nest with at least one pup. Nest building was defined as collecting bedding or nesting material and bringing it to the nest and shaping it into a new nest. Food-deprived mice were classified as aggressive (Agg+) or non-aggressive (Agg−) as follows: after an initial chemoinvestigation and grooming phase, Agg− animals exhibit non-aggressive behaviours such as pup retrieval, nest building, rearing and digging (Extended Data Fig. 1). Agg− animals were further classified as ‘parental’ or ‘ignoring’ based on whether initial chemoinvestigation and grooming were followed by parental behaviour components. Parental animals retrieved pups after a brief grooming period. Once in the nest, they remained with the pups, crouched above them and engaged in grooming and occasionally nest building (Extended Data Fig. 1p,q). By contrast, ignoring animals only performed non-pup-related behaviours—such as rearing and digging—after initial chemoinvestigation and grooming (Extended Data Fig. 1r,s). Aggressive contact was defined as close interactions with pups involving rapid, rhythmic head movement, biting or aggressive carrying of pups around the cage62,63. In behavioural experiments, if a pup was attacked, all pups were immediately removed, and the trial was terminated. During in vivo imaging experiments, if any pup was attacked, attacked pups were promptly replaced with new pups to enable the observation of multiple aggression episodes. In the rare event of injury, affected pups were immediately euthanized.
Prey assay
House crickets (Gryllus domesticus, 12–20 mm in length, purchased from the Northampton Reptile Centre) were used as targets. Immediately after pup-directed behaviour assays, a cricket was placed in the cage for 15 min. Capturing, biting or biting with forepaw assistance was classified as prey-directed aggression.
Residence intruder assay
Male or female adult mice 8–14 weeks of age were introduced into the resident’s cage immediately after the pup-directed behaviour assays in randomized order, and resident mice were allowed to interact with the intruder for 15 min. Trials in which the intruder exhibited aggression towards the resident were excluded. Mice were categorized as aggressive towards the intruder if biting and fighting occurred.
Elevated-plus maze test
A standard elevated-plus maze with two closed and two open arms, elevated 90 cm above ground, was used64. The assay was initiated by placing the mouse in the open arm of the plus maze, and animal trajectories were recorded for 10 min. Videos were captured and analysed using EthoVision XT 14 (Noldus).
Open-field test
A white behaviour test box (60 × 60 × 30 cm, length × width × height) was virtually divided into a centre (30 × 30 cm) and a periphery. A mouse was placed in the periphery and recorded for 10 min to measure the time spent in the centre or peripheral area. Videos were captured by a top camera and analysed using EthoVision XT 14 (Noldus). Custom detection profiles were set for each mouse, and the detection threshold was adjusted so that the mouse could be detected in >95% of video frames. The time spent in the closed versus open arm, and centre versus periphery, average speed and total time spent moving were quantified using the EthoVision animal tracking pipeline.
Food intake
Mice were single-housed for 4 days before food intake was measured. On the day of measurement, animals were provided with fresh bedding to avoid leftover food crumbs in the cage. A Petri dish with food pellets was provided and 1 h of food intake was quantified by calculating the weight difference of the Petri dish. Food intake on behavioural testing days was measured immediately after pup interactions. Baseline food intake was quantified on the day before behavioural testing during the same circadian time (4 h before the end of the dark phase) in sated mice. For refeeding, Agg+ mice were provided with food ad libitum for 1 or 2 h before pup interactions were assessed.
FOS mapping
To identify brain areas that are differentially recruited between aggressive and non-aggressive pup interactions in food-deprived mice, pup-directed behavioural assays were performed as described above (see the section ‘Pup-directed behaviour assay’). At 90 min after the first pup contact, mice were deeply anaesthetized and rapidly transcardially perfused with 30 ml ice-cold PBS, followed by 30 ml ice-cold paraformaldehyde (PFA) (4% in PBS). Brains were dissected and post-fixed in PFA (4% in PBS) at 4 °C for 16 h. The next day, brains were rinsed with cold PBS and 60 µm coronal sections were prepared with a vibratome (Leica VT1000 S). Sections were further post-fixed in PFA (4% in PBS) at room temperature for 10 min and immunostaining against FOS was performed (see the section ‘Immunohistochemistry’). Brain sections were imaged on a slide scanner, and FOS+ cell densities were quantified between sections from Agg+ and Agg− mice using QuPath software (see the section ‘Imaging’).
Mass spectrometry
Trunk blood was collected into EDTA tubes and samples were centrifuged at 2,000g for 10 min at 4 °C using a microcentrifuge. The supernatant (serum) was pipetted into a fresh 1.5-ml tube and samples stored at −80 °C. Next, 10 µl of serum was mixed with 30 µl ice-cold methanol to induce protein precipitation. Samples were briefly vortexed, placed on ice for 5 min and centrifuged at 4 °C for 10 min. Next, 30 µl of extract was mixed with 270 µl methanol, and 30 µl of the diluted extract was transferred to a vial equipped with an insert, followed by the addition of 1 nmol Scyllo-inositol (Sigma). Samples were dried and derivatized with 20 µl freshly prepared methoxyamine (20 mg ml–1, in pyridine) (both Sigma) at room temperature for >10 h, followed by a second step of derivatization with 20 μl BSTFA + 1% TMCS (Sigma) performed at room temperature for 1 h. Data acquisition was performed largely as previously described65 using an Agilent 7890B-7000C GC-MSD in EI mode. GC–MS parameters were as follows: carrier gas, helium; flow rate, 0.9 ml min–1; column, DB-5MS (Agilent); inlet temperature, 270 °C; temperature gradient, 70 °C (2 min), ramp to 295 °C (12.5 °C min–1), ramp to 320 °C (25 °C min–1, 3 min hold). The scan range was m/z = 50–550. Data analysis was performed using MANIC software (v.3.0.20)66. Metabolites were identified and quantified by comparing to the authentic standard of ghrelin (Anaspec AS-24160).
Oestrous cycle staging
Vaginal smears were taken immediately after pup interaction assays. Animals were scruffed and 20 µl of PBS was gently pipetted several times at the surface of vagina. Samples were air-dried and stained with 10 µl crystal violet (C.I. 42555, Merck). Mouse identifiers were shuffled, and the oestrous cycle was assessed by an individual blind to aggression phenotype (see ref. 67).
Histology and immunostaining
Perfusion and tissue sectioning
Animals were transcardially perfused with PBS followed by 4% PFA in PBS. Brains were dissected and post-fixed in 4% PFA overnight at 4 °C then washed in PBS. After embedding in 4% low-melting point agarose (Thermo Fisher, 16520-050) in PBS, 60-µm coronal sections were cut on a vibratome (Leica) and mounted on Superfrost Plus slides (VWR, 48311-703) with DAPI-containing Vectashield mounting medium (Vector Laboratories, H-1200). Acute, 250-µm-thick brain sections from electrophysiological recordings were post-fixed in 4% PFA in PBS with 200 mM sucrose (Sigma-Aldrich, S5016) and 0.1 M HEPES (Sigma-Aldrich, H3375) at 4 °C on a nutator overnight, rinsed in PBS and washed in PBS-T (0.3% Triton X-100 in PBS) for 1 h.
Immunohistochemistry
Immunostaining was performed in 48-well tissue culture plates. Brain sections were permeabilized for 30 min in PBS-T (0.3% Triton X-100 in PBS), post-fixed with 4% PFA in PBS for 10 min and washed in PBS (3× 20 min). Blocking was carried out for 3 h at room temperature in blocking buffer (3% BSA, 2% normal donkey serum in PBS). Incubation with primary antibodies (in PBS) was performed for 24–48 h on a nutator at 4 °C. After washing in PBS (3× 20 min), secondary antibodies were added in PBS-T for 48 h at 4 °C. After final washes in PBS-T (3× 20 min), sections were mounted. The following primary antibodies were used: rabbit anti-FOS (Synaptic Systems, 226003, 1:2,000); rabbit anti-NPY (Abcam, ab30914, 1:500); and rabbit anti-AgRP (Abcam, ab254558, 1:500). The following secondary antibodies were used: donkey anti-rabbit Alexa Fluor-568 (Thermo Fisher, A-11057, 1:2,000); donkey anti-rabbit Alexa Fluor-647 (Thermo Fisher, A-21245, 1:2,000); and goat anti-rabbit Alexa Fluor-647 (Thermo Fisher, A-21244, 1:1,000).
In situ hybridization
Animals were transcardially perfused with ice-cold PBS, and freshly dissected brains were embedded in OCT (Tissue-Tek, 4583), frozen on dry ice and stored at −80 °C. Subsequently, 18-µm cryosections were cut on a Leica CM1950 cryostat and collected on Superfrost Plus slides (VWR, 48311-703) in three series, only one of which was stained and imaged. Slides were fixed in 10% neutral buffered formalin, followed by a series of dehydration steps in ethanol (5 min each of 50%, 70%, 100% and 100% v/v ethanol). Slides were pretreated with RNAscope protease III reagent for 30 min at 40 °C. Single-molecule fluorescent in situ hybridization was performed on slides using a RNAscope LS Multiplex Reagent kit (Advanced Cell Diagnostics), a LS 4-Plex Ancillary kit and a Multiplex Reagent kit on a robotic staining system (Leica BOND-III). RNAscope probes were Hcn1 (ACD, 423658), Hcn2 (427009), Hcn3 (551528) and Hcn4 (421278). Immunostainings against the neuronal marker NeuN were subsequently performed (Millipore, MAB377, 1:500).
Imaging
Images were acquired on a Vectra Polaris Automated Quantitative Pathology Imaging system (Akoya Biosciences) at ×20 magnification. Regions of interest (ROIs) were selected using Phenochart software (Akoya Biosciences) and image tiles were spectrally unmixed using inForm Tissue Analysis software (Akoya Biosciences). Stitching of spectrally unmixed image tiles and image analyses were performed in QuPath software68. FOS-positive nuclei (or NeuN-positive neuronal cell bodies) were first detected using custom QuPath scripts. Detection of Esr1, Pgr and Hcn transcripts was subsequently performed on cell body detections. Thick brain sections (250 µm) were imaged on an upright confocal microscope (Zeiss LSM 710) using a ×63 (NA 1.4) oil-immersion objective and a z step size of 0.5 µm.
Surgical and recording procedures
Analgesia was provided 1 day before surgery (0.15 ml carprofen in 200 ml drinking water). Mice were anaesthetized using isoflurane (4% for induction, 1.5% for maintenance) in oxygen-enriched air and head-fixed in a stereotactic frame (Model 940, Kopf Instruments). Meloxicam (10 mg kg–1 body weight) and buprenorphine (0.1 mg kg–1 body weight) were given subcutaneously before craniotomy. The surgery site was closed using Vicryl sutures (Ethicon) or Vetbond surgical glue (3M). Carprofen was provided in drinking water for 2 days after surgery for postoperative pain management. Eyes were protected with ophthalmic ointment (Viscotears, Alcon). The rectal body temperature was maintained at 37 °C during surgery using a heating pad (Harvard Apparatus) and animals were kept in a heated recovery chamber until fully mobile. Animals were allowed to recover for at least 2 weeks before behavioural testing.
Brain coordinates
See Supplementary Table 1 for injection, implantation and recording coordinates. Coordinates are anteroposterior/mediolateral/dorsoventral and in mm. Dorsoventral coordinates are measured from the brain surface. Chemogenetic effectors were injected into two rostrocaudal Arc coordinates (−1.4/±0.25/−5.90 and −1.6/±0.25/−5.90 mm) to maximize the number of transduced neurons. For projection-specific Npy and AgRP knockdown, MPOA coordinates were adjusted to 0.0/±0.3/−5.05 mm to maximize the number of retrogradely labelled ArcAgRP neurons.
Chemogenetics
For chemogenetic activation, 200 nl AAV5-hSyn-DIO-hM3Dq(Gq)-mCherry (Addgene, 44361, 2.5 × 1013 genome copies (GC) per ml) or AAV5-hSyn-DIO-mCherry (Addgene, 50459, 1.8 × 1013 GC per ml) was injected into the Arc (see Supplementary Table 1 for coordinates). After assessment of spontaneous pup-directed behaviours 3 weeks after viral injection, CNO (Bio-Techne 12352200, 3 mg kg–1) was intraperitoneally injected, and pup-directed behaviour was assessed 30 min later. For chemogenetic inhibition, 250 nl AAV5-loxP-hGlyAG-2A-nlsVenus (1.6 × 1013 GC per ml, Crick Vector Core) was prepared from a pAAV-loxP-hGlyAG-2A-nlsVenus plasmid14,69 (a gift from H. Fenselau) and injected into the Arc (see the section ‘Brain coordinates’). Ivermectin (5 mg kg–1, dissolved in 7:3 propylene glycol and glycerol) was injected 24 h before the start of food deprivation, and behaviour was assessed after 6 h of food deprivation.
Optogenetics
To optogenetically activate ArcAgRP projections, 250 nl AAV5-EF1a-DIO-ChR2-EYFP or AAV1-EF1a-DIO-ChR2-EYFP (Addgene, 20298, 0.7 × 1013 GC per ml) or AAV1-EF1a-DIO-YFP (Addgene, 27056, 2.5 × 1013 GC per ml) was injected into the Arc (see Supplementary Table 1 for coordinates). During the same surgery, optic fibres (Doric Lenses) were implanted 200–400 µm above the target area (MPOA: dual fibre cannula 200/245 µm, 0.37 NA, GS1.0; LPOA: dual fibre cannula 200/245 µm, 0.37 NA, GS2.0; PVH: mono fibre cannula 400/470 µm, 0.37 NA). After 2–3 weeks of recovery, animals were connected to matching patch cords connected to a laser (Stradus 473–80 nm, Vortran) through a commutator (RJ1, Thorlabs). Four distinct protocols for optogenetic stimulation were used: acute stimulation whenever animals were close to a pup; or 15, 30 or 60 min of pre-stimulation followed by a 15-min pup-directed behaviour assay. A period of 3–4 days was allowed between two consecutive optogenetic experiments to prevent sensitization to pups. The light power exiting the fibre tip corresponded to an irradiance of 4.68 mW mm−2 at the target region (http://www.stanford.edu/group/dlab/cgi-bin/graph/chart.php). For acute stimulation, blue light was delivered in 20-ms pulses at 20 Hz for 1–4 s whenever the animal contacted a pup with its snout. In the pre-stimulation protocols, cycles of 1 s of 20 Hz stimulation followed by 4 s without stimulation were delivered for the indicated duration15.
Hormone receptor KO
AAV2/5-CMV-EGFP-Cre (250 nl, Addgene, 105545, 2 × 1013 GC per ml) was injected into the MPOA (see the section ‘Brain coordinates’) of Esr1loxP or PrloxP mice. Animals were tested 3 weeks after injection, and brain slices were subsequently prepared for histological analyses. The efficiency of viral-genetic receptor KO was established in a separate experimental cohort of Esr1loxP or PrloxP animals that received unilateral MPOA injections of either AAV2/5-CMV-EGFP-Cre or AAV2/5-CMV-EGFP (250 nl, Addgene 105530, 2 × 1013 GC per ml), and which has since been published3.
Gene knockdown
Constructs for shRNA-mediated knockdown of Npy and Agrp were developed using the Broad Institute’s hairpin design tool (https://portals.broadinstitute.org/gpp/public/seq/search) on the Npy (NM_023456.3, position: 3728–3748) and Agrp (NM_007427.3, position: 187–648) coding sequences. The following sequences were used: (1) Npy_817 CACTGATTTCAGACCTCTTAACTCGAGTTAAGAGGTCTGAAATCAGTG TTTTT; (2) Npy_818 GCTCTGCGACACTACATCAATCTCGAGATTGATGTAGTGTCGCAGAGCTT TTT; (3) Agrp_50 GTTCCCAGGTCTAAGTCTGAACTCGAGTTCAGACTTAGACCTGGGAACTT TTT; (4) Agrp_51 GGCAGGGGATGAGAATAAACTCGAGTTTATTCTCATCCCCTGCCTTTTT; (5) Agrp_4 GGCAAAGATCAGCAAGCAACTCGAGTTGCTTGCTGATCTTTGCCTTTTT (where TTTTT indicates the termination signal). Using NEBuilder, these oligonucleotides were cloned into the HpaI/SpeI sites of pAAV-G-Creon shRNA[Control] plasmid (Addgene, 181824)70, which generated the constructs pAAV-G-CreON-shRNA_817-NPY-GFP, pAAV-G-CreON-shRNA_818-NPY-GFP, pAAV-G-CreON-AGRPshRNA-GFP-50, pAAV-G-CreON-AGRPshRNA-GFP-51 and pAAV-G-CreON-AGRPshRNA-GFP-4, respectively. As a negative control, a scrambled sequence (CCTAAGGTTAAGTCGCCCTCGCTC GAGCGAGGGCGACTTAACCTTAGGTTTTTT) was designed using VectorBuilder.
pAAV-G-CreON-shRNA_817-NPY-GFP, pAAV-G-CreON-shRNA_818-NPY-GFP, pAAV-G-CreON-AGRPshRNA-GFP-50, pAAV-G-CreON-AGRPshRNA-GFP-51 and pAAV-G-CreON-AGRPshRNA-GFP-4 (see above) were packaged as rAAV2-retro capsids and the titre was measured by qPCR. For projection-specific knockdown of Npy, 400 nl of a 1:1 mix of AAV-retro-G-CreON-shRNA_817-NPY-GFP (3.8 × 1013 GC per ml) and AAV-retro-G-CreON-shRNA_818-NPY-GFP (2.3 × 1013 GC per ml) was bilaterally injected into the MPOA (see the section ‘Brain coordinates’). For projection-specific knockdown of Agrp, 400 nl of a 1:1:1 mix of AAV-retro-G-CreON-AGRPshRNA-GFP-50 (1.0 × 1013 GC per ml), AAV-retro-G-CreON-AGRPshRNA-GFP-51 (1.6 × 1013 GC per ml) and AAV-retro-G-CreON-AGRPshRNA-GFP-4 (1.3 × 1013 GC per ml) was bilaterally injected into the same coordinates. As control, AAV-retro-CreON-shRNA-scr expressing a scrambled shRNA (400 nl, 1.78 × 1013 GC per ml) was injected. Behavioural testing and/or electrophysiological recordings were performed 3 weeks after injection.
Cannulation experiments
Mice were implanted with stainless-steel bilateral guide cannulas (C235GS-5- 1.0/SPC, Protech International) 0.2 mm above the MPOA. Cannulas were fixed to the skull with dental cement. Dummy cannulas (C235DCS-5/SPC, Protech International) were inserted into guide cannulas to prevent clogging and closed with a dust cap. Mice were allowed to recover for 4 days. One hour before behavioural testing (see the section ‘Pup-directed behaviour assay’), 1 µl of ZD-7288 (Tocris 1000; 1 mM, in sterile artificial cerebrospinal fluid (ACSF)) or ACSF alone (vehicle) was administered to each side of the cannula at a rate of 0.5 µl min–1.
Fibre photometry
AAV-hsyn-DIO-GCaMP7s (Addgene, 104491-AAV1, 300 nl, 1.5 × 1013 GC per ml) was injected into the Arc of Agrp-cre mice and a 200 µm fibre-optic cannula (MFC_200/230-0.37_6mm_MF1.25_FLT, Doric Lenses) was implanted into the MPOA (see Supplementary Table 1 for coordinates). The cannula was fixed to the skull using UV light-curable glue (RelyX Unicem, 3M) and Superbond cement (Prestige Dental). Recordings were performed 3 weeks after surgery using a FP3001 fibre photometry system (Neurophotometrics). In brief, two LEDs (415 nm and 470 nm, light power of about 50 µW) were pulsed at 20 Hz in an interleaved manner to obtain an isosbestic motion signal (415 nm) and GCaMP activity (470 nm). A FLIR 277 BlackFly CMOS camera was used to detect fluorescent signals, and acquisition was controlled (and synchronized to the acquisition of behavioural video recordings) using Bonsai.
Miniature microscopy imaging
AAV2/1-syn-GCaMP7s (Addgene, 104487, 100–200 nl, 2 × 1013 GC per ml) was unilaterally injected into the MPOA of C57BL/6J mice using a Nanoject II or Nanoject III injector (Drummond Scientific) and pulled glass capillaries (3-000-203-G/X, Drummond Scientific). See Supplementary Table 1 for injection and implantation coordinates. After letting the virus diffuse for 5 min, the injection needle was slowly retracted and an integrated gradient-index lens (0.6 × 7.3 mm, 1050-002177, Inscopix) was slowly implanted and fixed to the skull using UV light-curable glue (RelyX Unicem, 3M) and Superbond cement (Prestige Dental).
Recordings started 6–8 weeks after surgery. Mice were connected to a miniature microscope (nVista, Inscopix) to check for sufficient expression of GCaMP7s. Imaging data were acquired using nVista HD software (Inscopix) at a frame rate of 20 Hz with 475 nm LED power of 0.1–0.2 mW mm–2, an analog gain of 5–8 and an image resolution of 800 × 1,280 pixels. Imaging parameters and focal depth were kept identical across sessions. Imaging and behavioural video collection were synchronized using Bonsai. Mice were connected to the microscope and allowed 20 min of habituation before recordings were performed in their home cage. A 1-min baseline was acquired before pups were introduced, which was used to calculate the relative fluorescence change for each ROI in the field of view.
Ex vivo electrophysiology
C57BL/6J mice were deeply anaesthetized with 3% isoflurane in oxygen and decapitated. The brain was quickly dissected and placed in ice-cold slicing solution containing (in mM): sucrose (214), KCl (2), NaH2PO4 (1.2), NaHCO3 (26), MgCl2 (2), CaCl2 (2) and d-glucose (10), equilibrated with carbogen (95% O2/5% CO2). Coronal brain slices (250 μm thick) containing the MPOA were cut on a vibratome (Leica VT1200S) in ice-cold slicing solution and transferred to an incubation chamber with ACSF containing (in mM): NaCl (127), KCl (2), NaH2PO4 (1.2), NaHCO3 (26), MgCl2 (1.3), CaCl2 (2.4) and d-glucose (10), which was continuously oxygenated with carbogen. After at least 1 h of recovery at 35 °C, slices were transferred to a submersion chamber under an upright microscope with infrared Nomarski differential interference contrast optics (Slicescope, Scientifica). During recordings, slices were submerged in, and continuously perfused (1–2 ml min–1) with, ACSF at near physiological temperature (33 °C) and continuously oxygenated with carbogen. Glass micropipettes (3–6 MΩ resistance) were pulled from borosilicate capillaries (World Precision Instruments) on a P-97 Flaming/Brown micropipette puller (Sutter) and filled with internal solution containing (in mM): potassium gluconate (140), KCl (10), KOH (1), EGTA (1), Na2ATP (2), Mg2ATP (2) and HEPES (10), pH 7.3, 280–290 mOsm. Access resistance was monitored throughout the experiment, and neurons in which it exceeded 25 MΩ or changed by ≥20% were excluded. The liquid junction potential was 16.4 mV and was not compensated. We characterized the intrinsic electrophysiological properties of cells using a standardized current-clamp protocol that consists of I/V curves, ramps and current injections. HCN-mediated voltage sag amplitudes were measured in response to hyperpolarizing 1-s direct-current steps71. T-type calcium currents were assessed using a standard current-clamp protocol in which cells were hyperpolarized to −120 mV and then stepped back to −60 mV72. The amplitude of the resulting rebound was then quantified. To assess excitability, ramping depolarizing currents (10 pA s–1) from +25 to +165 pA were injected. Spontaneous postsynaptic currents (sPSCs) were detected using a threshold-based detector (WinEDR v.4, template mode). The rise time was defined as the time needed for sPSC amplitudes to reach 1-e−1 (≈63%) of its maximal value, and the time constant of decay was defined as the time needed for the sPSC amplitude to return to 1/e (≈37%) of the resting state. The HCN channel blocker ZD-7288 (Tocris 1000) was added at a concentration of 50 µM 1 h before recordings. NPY (Phoenix Pharmaceuticals 049-03) was added at a concentration of 100 µM 1 h before recordings. NPY receptor antagonists (NPY1R: 10 µM BIBP 3226, Tocris, 2707; NPY2R: 100 nM BIIE 0246, Merck, SML2450) were added 1 h before recording73,74. Recordings were acquired using a Multiclamp 700B amplifier (Molecular Devices), low-pass filtered at 10 kHz and digitized using a Digidata 1550B digitizer (Molecular Devices). Slow and fast capacitive components were semiautomatically compensated. Offline data analysis was performed with Clampfit 10 software (Molecular Devices), WinEDR (v.4), WinWCP (v.5; http://spider.science.strath.ac.uk/sipbs/software_ses.htm) and custom routines written in Python (v.3.7).
Channelrhodopsin-assisted connectivity mapping
For channelrhodopsin-assisted connectivity mapping75, 200 nl AAV1-EF1a-FLEx-hChR2(H134R)-EYFP (Addgene, 20296, 7 × 1012 GC per ml) was bilaterally injected into the MPOA of Agrp-cre mice. Acute brain sections were prepared 3 weeks after viral injection. We used a CsCl-based internal solution containing (in mM): CsCl (140), EGTA (1), Na2ATP (2) and HEPES (10), pH 7.3, 280–290 mOsm. Spontaneous inhibitory postsynaptic currents were recorded in voltage-clamp configuration at −70 mV in the presence of 1 µM TTX (Alomone T-550) and 100 µM 4-AP (Sigma 275875). Drugs were washed in at least 10 min before recordings. Photostimulation was delivered from a 490 nm LED (pE-100, CoolLED) through a ×60 objective and consisted of 2–10 ms of light pulses at a light intensity of about 2.6 mW mm–2.
Quantification and data analysis
Error bars, exact n values and statistical tests are described in the figure legends. No statistical methods were used to predetermine sample sizes. Sample sizes were estimated on the basis of previous experiments performed in our group and are consistent with those generally used in the field. Animals were only excluded if viral transduction was unsuccessful or off-target or if the fibre, cannula or lens tip placement was off-target. For electrophysiological recordings, only cells with a stable series resistance of <30 MΩ were analysed. These criteria were determined before statistical tests were performed. The following experiments were replicated twice by different experimenters: switch to pup-directed aggression induced by 6 h of food deprivation and slice physiology recordings across the oestrous cycle. All attempts at replication were successful. Animals were randomly assigned to treatment and control groups. Experimental groups consisted of multiple cohorts to avoid litter and cage effects. Data acquisition was not performed blind. Behavioural data were scored by an individual blind to the experimental design, and analyses of behavioural, histological, electrophysiological and in vivo imaging data were conducted under blind conditions. Exact P values, t values, F values and degrees of freedom are provided in the source data.
Calculation of behavioural transition probabilities
To calculate the behavioural transition probabilities shown in Extended Data Fig. 1, we first created temporally ordered lists of scored behaviours for individuals classified as Agg+, parental Agg− or ignoring Agg−. We filtered these lists to include only relevant behaviours, then parsed them into sequential behaviour pairs (for example, behaviour 1→behaviour 2). For each unique pair, we calculated the transition probability by dividing the number of occurrences of that pair by the total number of transitions originating from behaviour 1. This produced a behavioural transition matrix for each individual mouse, whereby each entry represents the conditional probability of transitioning from one behaviour to another. Rows were normalized such that each value reflects the likelihood of transitioning to a new state given the current behaviour. For visualization, we constructed directed graphs in which nodes represent individual behaviours, arrows denote transitions and arrow thickness corresponds to the transition probability.
Calculation of predicted baseline switching rates
The observed switching rates of animals with hormone receptor ablation were compared with the predicted baseline switching rate, which would be expected for each cohort if receptors were intact. These baseline rates were determined using hypothesis testing on Poisson binomial distributions, which were constructed on the basis of the oestrous cycle distribution of each cohort using the poibin package (https://github.com/tsakim/poibin). The predicted switching rate corresponds to the mean of each custom Poisson binomial distribution.
Image analysis and registration
The ImageJ plugin ABBA76 was used to register coronal brain sections to the Allen Brain Atlas (CCFv3)77. In brief, x and y rotations were adjusted across all sections from a given brain, and two rounds of affine registration using Elastix were performed. Samples then underwent non-rigid registration using the BigWarp tool (sample channel: DAPI; atlas channel: Nissl). Positive cell detection was performed on the transformed samples using QuPath, followed by subcellular detection of Hcn transcript spots and clusters. Spot counts in clusters were estimated by dividing the cluster area by the expected size of individual spots. Transformed cell detections were exported from QuPath, visualized using a custom Python app (https://github.com/nickdelgrosso/ABBA-QuPath-RegistrationAnalysis) and analysed using custom scripts in Python (v.3.7).
Quantification of Npy and Agrp knockdown efficiency
Brain sections from Agrp-cre mice injected with conditional AAVs expressing GFP and shRNA targeting either Npy or Agrp (or a negative control, see the section ‘Gene knockdown’) were immunostained for NPY or AgRP, respectively (see the section ‘Immunohistochemistry’) and imaged using a Zeiss LSM 710 confocal microscope. Quantification was performed using pixel-based analysis, as NPY and AgRP immunoreactivity was primarily localized to fibres rather than cell bodies. Image stacks were imported into ImageJ, and the JaCoP plugin78 was used to calculate the percentage of pixels in the NPY or AgRP channels that colocalized with GFP-positive pixels.
Coexpression analysis
To assess coexpression of Hcn subunits (Hcn1 and Hcn2), Npy receptor genes (Npy1r and Npy2r) and Esr1 and Pgr in MPOA neurons, we analysed a previously published single-cell RNA sequencing dataset79. We queried the adult hypothalamus dataset (WMB-10Xv3-HY-log2.h5ad) and filtered for neurons assigned to the MPOA. Coexpression was assessed by calculating the proportion of cells expressing each gene above a defined threshold (1 copy; Extended Data Fig. 7h), and the overlap across marker-defined neuronal clusters were examined.
Processing and analysis of fibre photometry data
The recorded interleaved trace was separated into isosbestic (415 nm) and calcium-dependent (470 nm) channels using custom Python routines. To correct for motion artefacts and baseline drift, a linear fit of the 415 nm signal was computed and subtracted from the 470 nm signal. To further correct for slow fluctuations such as photobleaching, a moving minimum baseline (20-s sliding window) was subtracted from the resulting trace. The relative fluorescence change was then calculated as \(\frac{\Delta F}{{F}_{{\rm{mean}}}}=\frac{F-{F}_{{\rm{mean}}}}{{F}_{{\rm{mean}}}}\) and normalized using min–max scaling. Manually scored behaviours were aligned to the activity traces through timestamps acquired in Bonsai.
Processing and analysis of in vivo imaging data
Pre-processing
Image frames were spatially downsampled to 400 × 540 pixels. Drift of the baseline signal over time was removed using a spatial bandpass filter with lower and upper cut-off spatial frequencies of 0.005 and 0.5 oscillations per pixel, respectively. Motion artefact correction was performed, and the relative fluorescence change ΔF/F0 for each pixel compared with the baseline was calculated as \(\frac{\Delta F}{{F}_{0}}=\frac{F-{F}_{0}}{{F}_{0}}\), where F0 is the mean fluorescence value of each pixel during the baseline period). Cell detection based on princicpal component analysis (PCA) or independent component analysis was performed using a mean ROI radius of 7–9 pixels in Inscopix Data Processing software. All automatically identified cells were manually verified to exclude false-positive detections, and cells not detected by the algorithm were manually added. Cell traces were deconvolved using OASIS80 with a model order of 1 and a spike SNR threshold of 3.0.
Longitudinal registration
Longitudinal registration of pre and post field-of-views was performed using Inscopix Data Processing software without session correlation for thresholding. The resulting aligned traces were manually quality-controlled. ROIs with irregular shapes or without activity transients were discarded.
Evoked activity and absolute tuning index
For population-averaged neural activity and absolute tuning indices, we analysed the first behavioural bout of each specified action per session. To reduce potential confounds from previous behaviour occurrences, or cumulative social experience, and to ensure that neural activity reflected the response to the behaviour of interest, we selected bouts in which no other overt behaviours occurred during the baseline period. This was straightforward for isolated chemoinvestigation events (for example, pup or intruder sniffing), but more challenging for behaviours typically embedded in behavioural sequences, such as pup grooming and aggressive contact. These behaviours are often preceded by pup-directed sniffing or grooming, and only a very small number of episodes occurred in complete isolation. Grooming-related and aggression-related traces were therefore not excluded based on baseline contamination but were still limited to the first bout per session to minimize experience-dependent effects.
The absolute tuning index measures how strongly the activity for each detected cell deviates from baseline during a behavioural event, incorporating both positive and negative activity changes. This index accounts for variability by considering the standard deviation of both the baseline and activity period. The baseline and activity windows used for z score and tuning index calculations were adapted for each behaviour based on the average duration of behavioural bouts (that is, ±2 s for pup sniffing and attacks, ±4 s for pup grooming, ±5 s for male intruder sniffing, and ±3 s for female intruder sniffing). Tuning indexes were calculated on the basis of these behaviour-specific windows, using the pre-event period as baseline and the post-event period as the activity window. The z scores were calculated using ±5 s from behaviour onset as z = x − µσ, where x = ΔF/F of the current timestamp, µ is the mean ΔF/F of the baseline period and σ is the standard deviation of the baseline period. Significant responses were called when the z scored ΔF/F of the baseline and activity periods were significantly different (using unpaired t-tests). Cells were thereafter categorized as exhibiting increased, decreased or unchanged evoked activity. The single neuron tuning index was derived from performing an unpaired t-test between the activity and baseline periods and represents the absolute t value. Only neurons exhibiting increased activity during behaviours were used for z score plots.
PCA
PCA was used to reduce the dimensionality of the neural data and to identify the primary sources of variance in each recorded pup interaction session. Before applying PCA, the activity of each recorded neuron was standardized to ensure comparability across different neurons. The standardized activity for each neuron in each session was computed as follows:
$${x}_{i}^{{\prime} }=\frac{{x}_{i}-{\mu }_{i}}{{\sigma }_{i}}$$
where xi represents the activity of the i-th neuron, µi is its mean activity across the entire dataset and σi is its standard deviation. To quantify relationships between neurons, we computed the covariance matrix C of the standardized data as follows:
$$C=\frac{1}{n-1}\mathop{\sum }\limits_{i=1}^{n}{({x}_{i}-\bar{x})({x}_{i}-\bar{x})}^{T}$$
where n is the total number of neurons and \(\bar{x}\) is the mean activity vector across all neurons. The eigenvalues and eigenvectors of C were then computed, with each eigenvector vj representing a PC and its corresponding eigenvalue λj indicating the variance explained by that component. The PCs were ranked by their eigenvalues, with higher-ranked components capturing more variance. Recording sessions were included in the analysis only if the first two PCs accounted for at least 70% of the total variance. These two PCs were then used to project the neural population activity into a lower-dimensional space for visualization.
PC distance calculation
For the calculation of PC distances, activity episodes of 5 s after behavioural onset were extracted from each neuron. All episodes were standardized before analysis. To quantify the similarity between two neural activity episodes, a and b, in the k-dimensional PC space (k = 2), we computed the pointwise Euclidean distance at each time point t as follows:
$$d({a}_{t},{b}_{t})=\sqrt{\mathop{\sum }\limits_{i=1}^{k}{({a}_{t,i}-{b}_{t,i})}^{2}}$$
where at = (at,1, at,2,…,at,k) and bt = (bt,1, bt,2,…, bt,k) are the projections of the episodes onto the reduced k-dimensional PC space at time t, at,1 and bt,1 represent the i-th PC of each episode at time t, and k is the number of retained PCs (chosen to account for 90% of the variance).
To obtain the total distance between the two episodes, the pointwise Euclidean distances were summed across all time points as follows:
$$D(a,b)=\sqrt{\mathop{\sum }\limits_{t=1}^{T}\mathop{\sum }\limits_{i=1}^{k}{({a}_{t,i}-{b}_{t,i})}^{2}}$$
where T is the total number of time points in the episode. This total distance, D(a,b), serves as a similarity measure of neural population activity between conditions (for example, pre and post), with larger values indicating greater divergence in activity patterns.
Multiclass SVM classification
A SVM classifier was used to categorize behavioural states (pup sniff, pup groom, lick aggressive and late aggression) based on the following data: (1) raw neural data, (2) PCA-reduced neural data and (3) shuffled neural data (control). All behavioural episodes of >2 s were selected. Behavioural labels were assigned unique numeric identifiers using LabelEncoder, whereas the neural activity data served as the feature matrix (X) and the behavioural labels as the target variable (y). To address class imbalances, the synthetic minority oversampling technique (SMOTE) was applied. The k-neighbour parameter was dynamically adjusted based on the size of the smallest class. If a class contained fewer than two samples, SMOTE was not applied and the dataset was excluded from the analysis. Classifier performance was evaluated over 50 iterations. The dataset was split into training and test sets (split by episode number rather than by frame number) using stratification to preserve class distributions. To avoid data leakage, splits were made based on episode numbers rather than frame numbers. The SVM classifier was implemented using SVC from scikit-learn with default parameters. After training, predictions were generated for the test set, and accuracy was computed as follows:
$${\rm{Accuracy}}=\frac{{\sum }_{i=1}^{n}{\mathbb{I}}(\,{y}_{{\rm{pred}},i}={y}_{{\rm{test}},i})}{n}$$
where ypred and ytest are the predicted and true labels, respectively, \({\mathbb{I}}\)(·) is the indicator function, which returns 1 if the predicted and true labels match, and 0 otherwise, and n is the total number of test samples.
The accuracy of the SVM trained on raw neural data, PCA-reduced neural data and shuffled data was then compared. To assess classification performance across behavioural states, a confusion matrix was computed. The average accuracy and average confusion matrix were obtained by summing the confusion matrices over all iterations and then normalizing them row-wise as percentages as follows:
$${{\rm{N}}{\rm{o}}{\rm{r}}{\rm{m}}{\rm{a}}{\rm{l}}{\rm{i}}{\rm{z}}{\rm{e}}{\rm{d}}{\rm{C}}{\rm{M}}}_{ij}=\frac{{{\rm{C}}{\rm{M}}}_{ij}}{{\sum }_{j=1}^{m}{{\rm{C}}{\rm{M}}}_{ij}}\times 100$$
where CMij represents the number of times a sample from class i was classified as class j, and m is the total number of classes.
HMM analysis
To determine the optimal number of states for the HMM, we first explored a range of state values and assessed model performance using the evidence lower bound (ELBO). ELBO, a standard metric in variational inference, measures model fit, with higher values indicating better performance. The optimal state number was identified by locating the turning point in the ELBO curve, beyond which additional states provided diminishing improvements. This optimal value (here 5; Extended Data Fig. 9v) was then used for all subsequent analyses. The HMM was implemented using the ssm package (https://github.com/lindermanlab/ssm) with a Gaussian observation model. This framework applies Bayesian learning and inference to state-space models and is well suited for analysing sequential neural data9. The HMM was trained on neural recordings using the expectation-maximization algorithm for 50 iterations, with initial state assignments determined using k-means clustering. To assess the relationship between neural states and behaviour, we constructed a binary matrix for each behaviour, marking timestamps where the behaviour occurred as 1 and all other timestamps as 0. We then identified the state most frequently associated with each behaviour and computed the conditional probability of that behaviour occurring in the inferred state. This probability quantifies how strongly a given neural state is linked to specific behavioural patterns.
Selectivity analysis
To assess the selectivity of individual neurons for a particular stimulus, social (pups, male intruder or female intruder) and non-social stimuli (Lego brick, bedding or food) were presented sequentially in randomized order. Selectivity for pups versus other stimuli was quantified using a choice probability approach4,81. For each neuron, fluorescence signals (ΔF/F0) recorded during pairs of chemosensory investigation behaviours (for example, pup versus intruder investigation) were used to estimate how reliably the two behaviours could be distinguished based on their ΔF/F0 distributions. Specifically, ΔF/F0 values were extracted for each neuron’s activity during behaviour α (for example, attack) and behaviour β (for example, sniff). These distributions were plotted as paired histograms and cumulative distribution functions (CDFs). A ROC curve was then generated, with the CDF of pup sniffing on the x axis. The selectivity index was computed as: Selectivity index = 1 − AUCROC.
Here AUCROC is the area under the ROC curve. Neurons exclusively active during pup sniffing have a selectivity index of 1, those exclusively active during investigation of another stimulus an index of 0, and nonselective neurons an index of 0.5. To minimize the effects of gradual desensitization, only the first chemosensory investigation episode for each stimulus was used.
For aggression-related analyses (Extended Data Fig. 9r), we adapted this approach to test for persistent neural encoding. Following the first pup-directed aggression episode, we extracted the ΔF/F0 values for each neuron during aggression periods and non-aggression periods. Selectivity indices were computed as above. To assess population-level structure, we analysed the distribution of selectivity indices across neurons: a normal distribution centred at 0.5 indicates persistent state encoding, whereas skewed distributions suggest selective responses to discrete behavioural events. For comparison, we applied the same analysis to pup sniffing and pup grooming behaviours using activity recorded before the first aggression episode. Selectivity distributions for sniffing and grooming were significantly skewed, which suggested event-related encoding by discrete neuronal subpopulations.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.