Strains and standard culturing methods
The strains and chemicals used are listed in Supplementary Tables 1 and 2. E. coli K-12 BW25113 pDGUV-GFP50 was cultured in LuriaâBertani broth (Sigma-Aldrich) supplemented with 100âµgâmlâ1 carbenicillin (Roth). R.âmicrosporus strain NH is R.âmicrosporus CBS 631.82; R.âmicrosporus strain EH is R.âmicrosporus ATCC62417. M.ârhizoxinica HKI-0454 and R.âmicrosporus were cultured as previously reported42 at 28â°C. M.ârhizoxinica was fluorescently labelled with cytosolic GFP using pBBR-P12-GFP51. R.âmicrosporus was grown on potato dextrose agar (ThermoFisher) at 28â°C with 20âµgâmlâ1 gentamicin. Respective antibiotics for plasmid retention were used as required for the culture of bacteria in all experiments, including the growth of bacteria inside the fungus, in which case, additionally, gentamicin was used to prevent extracellular bacterial growth.
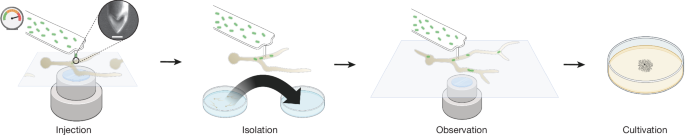
Bacterial injections with FluidFM
The basic setup of the instruments, FluidFM probe processing steps, probe cleaning and probe coating were as previously described36, but the pressure was controlled with a FlowEZ 7000 (Fluigent), in the range of 0â7,000 mbar, using an AT550-9L compressor (WenLing) as pressure source. The probe apex was sharpened with a Helios 5UX DualBeam focused ion beam scanning electron microscope (ThermoFisher) as described previously36,52, but to a new shape that resulted in a sharp double point, as shown in Fig. 1, by targeting the probe from the front and shaping a centred point with a 60° central angle. The cantilevers of the used probes had a nominal stiffness of 1.6âNâmâ1. The fungal sample was prepared by seeding spores in 50-mm WillCo glass-bottom dishes (WillCo Well) and adding 4âml potato dextrose broth (PDB) medium (ThermoFisher) containing 34âµgâmlâ1 chloramphenicol, incubating at room temperature for 14â16âh, washing 1â3 times with 4âml PDB, depending on germling concentration, exchanging the medium for protoplasting mix (to soften but not completely disintegrate the cell wall; 1.6âg Cellulase Onozuka R10 (Duchefa Biochemie), 40âmg chitinase (Merck), 40âml iced MMB (0.5âM mannitol, 0.05âM maleate pHâ5.5), filtered through a 0.22-µm syringe filter), and incubating for another 3â5âh at room temperature. If germlings were too dense, the sample supernatant was filtered through a 0.22-µm syringe filter. The E.âcoli sample was grown in a 10-ml culture in a baffled shake flask overnight at 37â°C, washed three times in Hepes2 buffer (10âmM HEPES, 150âmM NaCl, pHâ7.4), and adjusted to an optical density of 2 at 600ânm. The M.ârhizoxinica sample was grown in a 2-ml culture in a 12-ml culturing tube for 3 to 5 days at 28â°C and prepared like the E. coli culture. Bacterial suspensions (15âµl) were pipetted into the reservoir of the FluidFM probe. The probe was moved towards the glass surface (zâ=â0âµm) beside the targeted germling and then retracted to a z-value of +8 to +10 µm. The injection site of the germling was chosen on the basis of direct proximity to the glass surface, preferring thick germtube portions. As force-controlled puncturing was not possible owing to the softness of the probe, the germling was punctured by advancing the probe by 10âµm (corresponding to a nominal z-value of +1âµm to â2âµm), holding this position for 5âs and retracting to a z-value of +1 to +3âµm. Successful puncturing of the germling could be monitored with wide-field illumination by observing cytoplasmic flow. Immediately following puncture of the germling, the microfluidic system was pressurized with 3â4âbar, stopping turgor-induced backflow into the probe. Subsequently, the pressure was increased to 6.5âbar until liquid flow into the germling was noticed, and then quickly reduced to 3â4âbar to prevent bursting of the germling. Injection of bacteria was confirmed by switching to the fluorescence channel, and then the pressure was slowly reduced to 0âmbar to allow recovery of the germling. The injected germling was isolated to a fresh dish filled with recovery medium (3.8âml MMB, 1âml PDB, 160âµl 4âM sorbitol) after 3â10âmin, or after the germling recommenced growth. Isolation was carried out by lifting the FluidFM and exchanging the sample dish for the recovery dish, with the germling sticking to the probe. Recovery of the germling and the ensuing dynamics were visualized using time-lapse and z-stack images in the wide-field and the fluorescence channels. Some of the injections were carried out with the addition of 1:1,000 calcofluor white (Merck) in the protoplasting mix and recovery medium to visualize the cell wall. Germlings were grown in recovery medium overnight and then detached from the probe using overpressure, lifting of the FluidFM and scraping with a plastic pipette tip. Mycelium was then transferred to a potato dextrose agar plate and incubated at 28â°C.
Spore collection
Spores were collected 6â±â1 days after injection or plating from spores. Spore solution (8.5% NaCl, 1% Tween 20) was added to plates (16âml for square plates; 12âml for round plates), and the spores were thoroughly detached using a spatula. The remaining mycelium was clumped up and gently pressed with the spatula to release the spore solution from the mycelium. The spore solution was filtered through a 10-µm CellTrics filter (Sysmex). Spores were washed three times with 1âml Hepes2 (relative centrifugal force of 8,000; 2âmin) and stored at 4â°C overnight for FACS, or in 50% glycerol at â20â°C (for working stocks) or â80â°C (for long-term storage).
Flow cytometry and cell sorting
Analysis and sorting of spores were conducted at the ETH Flow Cytometry Core Facility on a FACSAria Fusion BSL2 cell sorter (BD). Single spores were selected using SSC-AâFSC-A and FSC-HâFSC-A gates. Colonization by bacteria was checked using an SSC-AâeGFP-A gate. (See Supplementary Fig. 1 for the gating strategy). For samples for which autofluorescence was suspected to complicate the positioning of the positive gate, an mCherry-A versus PerCPâCy5-5-A channel was used to check for autofluorescence but was not included in the gating. For determination of the fraction of positive spores, 100,000 to 1,000,000 spores were analysed depending on the size of the fraction. For bulk sorting, spores were sorted into 1.5-ml Eppendorf tubes. For determination of germination success, single spores were sorted into 96-well plates containing 125âµl PDBâ+â34âµgâmlâ1 chloramphenicol per well. For verification of positive gates, bulk-sorted spores were intermittently checked under the microscope. Collected data were analysed using FlowJo v10 software (BD). For sorting of high, medium and low fractions of positive spores, gates were set qualitatively in the positive population as illustrated in Fig. 4b.
Determination of germination success
Single-spore-sorted 96-well plates were incubated at 28â°C and checked visually with a Zeiss SteREO Discovery.V8 microscope (Zeiss) for the appearance of a germling. Per sample, three plates of positive spores and one plate of negative spores were sorted. In round 1 of the adaptive laboratory evolution experiment, five positive plates were sorted per sample. Germlings were counted 1 day and 2 days after sorting, after which point there are no new germlings to be discovered. For positive plates, five germlings were checked microscopically on day 1 to confirm the presence of fluorescent bacteria. If a germling could not be confirmed to have endobacteria, five more germlings were checked. No samples failed this control by having more than 20% of germlings without easily detected endobacteria. To calculate the percentage of delayed germinations, the percentage of germlings not detected on day 1 but detected on day 2 was calculated (with 100% corresponding to the number of germlings detected on day 2).
Bacterial isolation
For isolation of M.ârhizoxinica from R.âmicrosporus, 50âml MGYM9â+â34âµgâmlâ1 chloramphenicol was inoculated with spores in a 500-ml baffled shake flask and incubated with shaking at 100âr.p.m. for 5â7 days. Build-up of mycelium on the wall was periodically flushed off by gentle shaking and tilting by hand. Once the medium became turbid, an inoculation loop was inserted in the medium, avoiding mycelial clumps and used for streaking out on agar plates. Plates were checked daily for 3 days and growing fungus was cut out if detected. Plates with bacterial colonies were further cultivated according to standard protocols and were used to make cryogenic stocks. Alternatively, a 2-µm syringe filter (Merck) was used to separate bacteria from mycelium and spores, and the filtrate was used for further cultivation in liquid.
Adaptive laboratory evolution experiment
For the evolution experiment, spores collected in the first round after injection were sorted for determination of germination success, and the remaining spores were bulk sorted for positive spores. The positive spores were split into 10 equal lines, resulting in approximately 300 spores per line. Thereafter, the ten lines were kept separate. The germination success and the fraction of positive spores were determined in every round for every line, and at every round after round 1, some positively and negatively sorted spores were cryopreserved, and bacteria were isolated from 10,000 positively sorted spores (approximately 300 for round 1). The product of the positive fraction and the germination success was calculated to give the fitness index, indicating the percentage of spores that would yield bacteria-populated germlings for the start of a next round without selection. Plating for the evolution experiment was carried out on 120âÃâ120âmm square Petri dishes (Greiner) to increase the available surface area. Spores to be plated were taken up in 100âµl of buffer, which was pipetted into five parallel lines with equal spacing on the plate. Standard spread plating for high spore numbers had previously proved to lead to inconsistent spore formation. From round 2 on, high densities of spores could be plated for the next round as the fraction of positive spores increased. For making round 3 plates, the highest number of spores was seeded, up to 830,000 spores per plate, whereas for rounds 4 and 5 the numbers varied slightly around 100,000, and from thereon 100,000 were used for all rounds and lines. The number of seeded spores is shown in the Source Data for Fig. 3a. After round 7, only the three best-performing lines according to the fitness index were grown individually (lines 2, 4 and 7), whereas the other seven lines were pooled to line P by mixing equal amounts of positive spores. A scheme indicating the population sizes of spores and the pooling regime can be found in Fig. 3a.
Detection of rhizoxin
A total of 10,000 positively sorted spores per line from round 7 were plated. Strain EH colonized by M.ârhizoxinica, the axenic strain NH and a liquid culture of M.ârhizoxinica served as controls. Plates were grown for 11 days and extracted with 50âml ethyl acetate with shaking at 100âr.p.m. overnight at 28â°C. The organic phase was separated, dried with sodium sulfate, filtered through paper filter, and evaporated with a rotary vacuum evaporator. Samples were taken up in 1âml acetonitrile, centrifuged at 20,000g for 10âmin and 800âµl of the supernatant was stored at â80â°C until liquid chromatography with tandem mass spectrometry (LCâMS/MS) analysis. LC separation was carried out with a Thermo Ultimate 3000 UHPLC system (Thermo Scientific) using a C18 reversed-phase column (Kinetex XB-C18 column, particle size 1.7âµm, pore size 100âà ; dimensions 50âmmâÃâ2.1âmm, Phenomenex). Solvent A was 0.1% (v/v) formic acid in water and solvent B was 0.1% formic acid in acetonitrile at a flow rate of 500âµlâminâ1. Solvent B was varied as follows: 0âmin, 25%; 3âmin, 90%; 5âmin, 90%; 5.3âmin, 25%; subsequently, the column was equilibrated for 2âmin at the initial condition. The injection volume was 2âµl.
MS-product reaction monitoring analysis was carried out with a Thermo QExactive plus instrument (Thermo Fisher Scientific) in the positive Fourier transform mass spectrometry mode. MS level 1 scans were carried out with a mass resolution of 35,000 (m/zâ=â200) and MS level 2 scans were carried out with a mass resolution of 17,500. Parent ions were isolated at m/z 594.34, 610.337, 612.3531, 626.3323 and 628.348 with unit resolution and fragmented by high-energy C-trap collision dissociation applying a normalized collision energy of 28âeV. A heated electrospray ionization probe was used with the following source parameters: vaporizer temperature, 380â°C; sheath gas, 50; auxiliary gas, 20; sweep gas, 0; RF level, 50.0; capillary temperature, 275â°C. See Supplementary Fig. 2 for relevant spectra.
Genomics
For the generation of sequencing samples of M.ârhizoxinica, bacteria were grown according to standard culturing conditions after isolation from the respective time point (Methods, Bacterial isolation), and 4 ml of sample adjusted to an optical density of 1 at 600 nm were pelleted by spinning at a relative centrifugal force of 11,000 for 1âmin. Genomic DNA was prepared using the MasterPure DNA Purification Kit (LGC). Genomic DNA was sent on dry ice to BMKGene (Biomarker Technologies) for further processing.
For generation of sequencing samples of R.âmicrosporus, mycelium was grown in 500âml malt extract broth (Thermo Fisher Scientific) in 2-l shake flasks for Illumina sequencing or in 1.5-l malt extract broth in 5-l shake flasks for PacBio sequencing at 37â°C for 5âdays, with the addition of gentamycin and chloramphenicol. The mycelium was then filtered on a 110-mm filter paper and washed thoroughly with double-distilled H2O and for Illumina samples additionally with 150âml of 70% ethanol. The mycelium was then removed from the filter paper, packed into a 50-âml screw cap tube and frozen in liquid nitrogen. The samples were then sent to BMKGene (Biomarker Technologies) for further processing.
For genome assembly of R.âmicrosporus CBS 631.82, Pacbio HiFi sequencing with RS II produced a total of 3,867,257,442âbase pairs (bp) in 353,300 reads of average length 10.9âkilobases and average quality score 30.3. The reads were assembled with Flye (v2.9.2)53 with the –pacbio-hifi flag, resulting in 118 contigs of total length 55,743,399âbp with an N50 (the shortest contig of the set of the largest contigs making up 50% of the assembly) of 1,370,944âbp. BUSCO (v5.4.7)54 was used to check the quality of the assembly, using the lineage dataset mucorales_odb10, giving the following result: C (complete): 97.5%, S (single-copy): 5.1%, D (duplicated): 92.4%, F (fragmented): 1.7%, M (missing): 0.8%, n (number of genes): 2,449. Thus, although nearly complete, the genome also seems to be largely duplicated. The assembly was gene-called with BRAKER (v3.0.6)55,56,57,58,59,60,61,62, using the âfungus flag, and then functionally annotated with eggNOG-mapper (v2.1.12)63 using the option –target_taxa Fungi. BUSCO reported a slightly improved completeness for the called genes: C: 99.8% [S: 1.4%, D: 98.4%], F: 0.1%, M: 0.1%, n: 2,449.
For calling mutations from the adaptive evolution experiment for R.âmicrosporus and M.ârhizoxinica, the short-read Illumina sequences provided by BMK were used. The resulting raw reads were cleaned by removing adaptor sequences, low-quality-end trimming and removal of low-quality reads using BBTools v38.18 (https://sourceforge.net/projects/bbmap/). The commands used for quality control can be found on the Methods in Microbiomics web page (https://methods-in-microbiomics.readthedocs.io/en/latest/preprocessing/preprocessing.html). Single nucleotide polymorphisms were called using two different toolsâSnippy and bcftools64. For variant-calling with bcftools, reads were first aligned to the PacBio assembly of R.âmicrosporus CBS 631.82Â or to the M.ârhizoxinica reference genome (GCF_000198775.1) using BWA-MEM v0.7 (ref. 65). Duplicate reads were marked and removed using GATK4 v4.2 (MarkDuplicates)66. Variants were called using the bcftools call command. Single nucleotide polymorphism calls were validated by comparing results obtained by two independent tools. All the variants that were detected in the ancestral sample were filtered out from all of the evolved samples using bcftools-isec to investigate variants that arose during the evolution experiment. The resulting VCF files were filtered using bcftools with the following criteria: -Ov -sLowQual -g5 -G10 -e âQUALâ<â200â||âDP4[2]â<â3â||âDP4[3]â<â3â||â(DP4[2]â+âDP4[3])/sum(DP4)â<â0.1â||âMQâ<â50â. Each variant call produced by each of the tools was manually checked by analysing the read alignments at variant positions using samtools pileup. Mutations were annotated using SnpEff67 and InterProScan45. STRING was used to identify putative biological processes by searching for interactions on the basis of the closest-related genes for fungi found in the STRING database46.
Fitness without selection pressure
Spores for round 0 were first collected from a plate grown according to the standard conditions from the adaptive laboratory evolution experiment. For this, spores from a fresh FAncâBAnc injection plate were bulk sorted and 100,000 positive spores were plated. For FEvoâBAnc, frozen positive spores from an injection plate were grown and then 100,000 positive spores were plated; for FEvoâBEvo, frozen spores from the evolution experiment from round 10 line 4 were grown and then 100,000 positive spores were plated. From these plates, spores of round 0 were collected. Then 100,000 spores were bulk sorted gating only for single spores but disregarding the GFP signal intensity of the spores and used for subsequent rounds. Every round, 1,000,000 spores per sample were analysed to determine the fraction of positive spores. The endosymbiosis was considered washed out once the positive fraction fell below the threshold of 1/100,000 spores, at which no positive spore is expected to be plated for the next round. The experiment was stopped after round 5. The theoretical trajectory of the positive fraction was calculated using the following formula:
$${p}_{x}=({p}_{x-1}\times g\times {p}_{0})/({p}_{x-1}\times g+[1-{p}_{x-1}]\times e),$$
where p is the positive fraction; x is the round for which the positive fraction is being determined; g is the germination success of positive spores as measured in round 0; e is the germination success of negative spores derived from the long-term average from the evolution experiment (69%); and p0 is the positive fraction measured in round 0 to describe how many spores formed by bacteria-positive germlings are again bacteria positive.
Determination of bacterial load
Spores sorted for high, medium and low intensity were imaged in the wide-field and GFP channel to create z-stacks. Overlays of 10 spores per population per sample are shown in Extended Data Fig. 2a,b and served as visual confirmation for the correlation of GFP intensity as measured by FACS and bacterial population size. Contrast and brightness were kept the same. The volume of voxels considered to be bacteria was calculated using Matlab2018a (Mathworks) using sections of published code36. To gauge the appropriate threshold, the three-dimensional rendering of the resulting voxel cloud was visually inspected, and the diameter of single particles was checked. An example of the resulting three-dimensional rendering of samples is shown in Extended Data Fig. 2c.
Spore longevity
Samples were grown as described in the section entitled Fitness without selection pressure. Single spores were sorted into 96-well plates with 75âµl of Hepes2â+â34âµgâmlâ1 chloramphenicol in each well. Per sample, 15 plates with negative spores and 15 plates with positive spores were sorted. Plates were then incubated at 16â°C. One day before the nominal time point, three plates per condition were activated by adding 125âµl of PDBâ+â34âµgâmlâ1 chloramphenicol to each well and incubating at 28â°C.
Statistical analyses
Statistical tests indicated in the figure legends were run with GraphPad Prism v9.0.0.
Images, videos, plots and figures
Images and videos were edited using Fiji68 for contrast, z-stacks, time stamps, overlays and scale bars, as indicated in the respective sections of the Methods and figure legends. Videos were cut together and labelled using iMovie v10.4 (Apple). Plots were generated using GraphPad Prism 9. Figures were assembled and edited using Adobe Illustrator 2020. Illustrations for Fig. 1 were created with BioRender.com.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.