Study approval
All animal care and experimental procedures were approved for mice by the Institutional Animal Care and Use Committees of the Korea Advanced Institute of Science and Technology (KAIST; KA2024-122-v1) and the University of Missouri (9797), and for M. fascicularis monkeys by the Korea Research Institute of Bioscience and Biotechnology (KRIBB-AEC-24079).
Animals
Prox1–GFP mice32 (FVB background, 8–12 weeks of age for adults and 80–105 weeks of age for aged mice) were bred and maintained under specific pathogen-free conditions at KAIST. Adult (8–12 weeks of age) and aged (80–95 weeks of age) C57BL/6J mice were purchased from JAX (USA) or the Animal Center of Aging Science of Korea Basic Science Institute. Mice received ad libitum access to a standard diet and water and were exposed to 12-h light–dark cycles at 23–24 °C and 40–60% humidity. Experiments were performed during the light period. Mice of both sexes were used for all experiments and were anaesthetized by intraperitoneal injection of urethane (1.5 mg kg−1) or a mixture of urethane (1.5 mg kg−1), ketamine (0.75 mg kg−1) and xylazine (0.3 mg kg−1) before procedures. Supplemental anaesthesia was given as necessary during procedures. Body temperature was maintained at 36.5–37.5 °C during surgical and imaging procedures.
M. fascicularis monkeys (7–14 years of age), maintained at the National Primate Research Center of the Korea Research Institute of Bioscience and Biotechnology, were housed individually to prevent physical contact and minimize potential harm during the experiment but had visual and auditory interactions with neighbouring monkeys. Cage dimensions (60 cm × 80 cm × 80 cm) followed the guidelines of the US National Institutes of Health. Monkeys were housed with 12-h light–dark cycles at 22–26 °C and 45–55% humidity and provided a commercial monkey diet (Teklad 2050, Envigo), assorted fruits, ad libitum water, and rubber and plastic toys for environmental enrichment. Monkeys were anaesthetized by intramuscular injection of ketamine (5 mg kg−1) and atropine (0.02 mg kg−1) before the procedures and supplemented as necessary during procedures. Veterinary monitoring adhered to the non-human primates research guidelines70.
Imaging of CSF outflow to scLVs
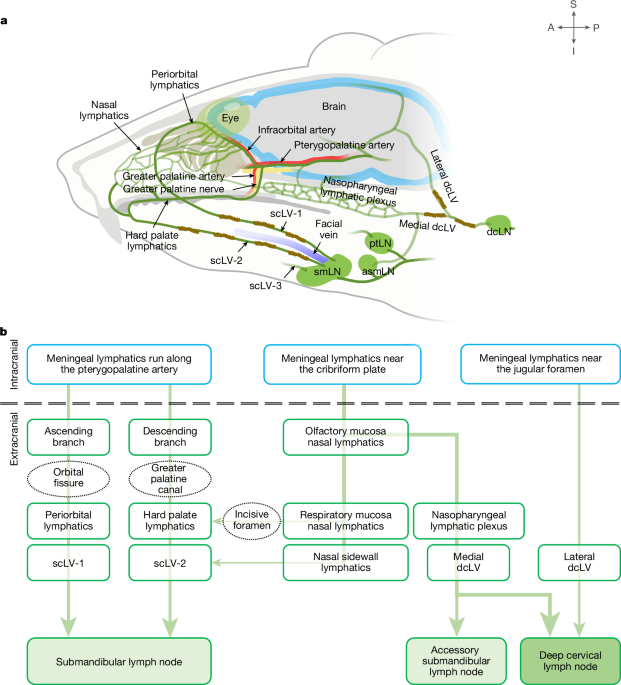
To assess CSF outflow through periorbital, nasal and hard palate lymphatics to scLVs and lymph nodes, 1 μl of phosphate-buffered saline (PBS) containing TMR–dextran (10 kDa, 50 mg ml−1; D1816, Invitrogen) was infused over 1 min into the SAS at the cisterna magna of Prox1–GFP mice23. Anaesthetized mice in the prone position were placed on a stereotaxic frame under a surgical microscope. The head was adjusted to a 90° angle to the body axis with the help of a mouthpiece to facilitate access to the cisterna magna. After the skin was incised in the midline of the posterior neck, the muscle layers were carefully separated with microscissors. The atlanto-occipital membrane overlying the cisterna magna was superficially penetrated using a 33-gauge NanoFil needle (World Precision Instruments). Then, 1 μl of PBS containing TMR–dextran was infused into the SAS over 1 min using a microsyringe (88000, Hamilton) and a micro-infusion machine (Fusion 100, Chemyx Inc.). The needle was left in position for 7 min and then slowly removed to prevent CSF leakage. The muscle layers and neck skin were then sutured with 6-0 black silk (SK617, Ailee). The abdominal aorta was cut to remove blood 15–120 min after the infusion, and the neck skin above the submandibular lymph node was removed under a fluorescence surgical microscope (SZX16, Olympus). Although the direction of TMR–dextran in lymphatics scLV-1 and scLV-2 that drained to the submandibular lymph node was observed, the skin overlying the nasal sidewall, medial cantus, cheek, chin and anterior auricular regions, including lateral canthus was carefully removed without damaging the lymphatics. Superficial cervical lymph nodes were exposed by dissecting the fascial layer and surrounding adipose tissue in the direction of lymphatics that drained from the submandibular lymph node to the parotid lymph node. Then, images of the lateral and ventral sides of the head and neck were obtained to record TMR–dextran in periorbital, nasal or hard palate lymphatics connected to cervical lymphatics. Images were captured with a fluorescence stereo zoom microscope (AxioZoom V16, ZEN 2.3 (blue) v2.3.64.0, Carl Zeiss) with a Plan-Neofluar Z ×1.0 objective lens and HE-GFP or Cy3 filter (Carl Zeiss). PBS was used to prevent tissue drying during dissection and imaging.
Infusion of fluorescent tracers in mice
One microlitre of FluoSpheres (diameter of 0.5 µm, polystyrene microbeads, carboxylate-modified surface, red fluorescent (580/605), 2% solids 98% DW; F8887, Thermo Fisher Scientific) or 1 μl of Qdot 705 (Q21361MP, Invitrogen) was infused over 1 min into the SAS of Prox1–GFP mice at the cisterna magna16,23,33. Subsequently, the head was removed for the histological analysis as described in section ‘Tissue preparation and immunohistochemistry’. One microlitre of Alexa-647-conjugated ovalbumin (O34784, Invitrogen) was infused into the mouth floor over 1 min to identify the initial region of the lymph drainage through scLV-3 to the submandibular lymph node. TMR–dextran (0.5 μl) was infused intradermally over 1 min into each of three facial regions: (1) nasal and medial canthus; (2) lateral canthus and anterior auricular; and (3) cheek and chin.
Ligation of cervical LVs
To determine the effect of blocking flow through dcLV or scLVs on CSF outflow to cervical lymph nodes, either the medial and lateral dcLV or scLVs were ligated bilaterally with 10-0 polypropylene suture (W2794, Ethicon) after the neck muscles were retracted in Prox1–GFP mice23. Sham controls underwent the same operation without the ligation. Two weeks later, TMR–dextran fluorescence in cervical lymph nodes and the nasopharyngeal route was measured 60 min after intracisternal infusion of TMR–dextran (1.0 μl).
Tracer infusion and intravital imaging in monkeys
The monkeys were positioned in the sphinx posture using a custom-built stereotaxic frame, and then FluoSpheres or indocyanine green71 were delivered by image-guided cisterna magna infusion under isoflurane anaesthesia (1.5% in 2 l min−1 oxygen). Placement of the needle tip within the cisterna magna was confirmed using XperCT imaging (Philips), and then the correct position was verified by CSF discharge. Oxygen saturation, heart rate, respiration rate and body temperature were monitored and maintained within normal ranges72. Ten minutes before infusion of the tracers, 1 ml of CSF was removed with a 23-gauge needle connected to a 1-ml Hamilton syringe via a Dual Removable Needle Coupler (Hamilton). After CSF removal, FluoSpheres or indocyanine green (5% in PBS; 1340009, Merck) were infused into the SAS at 250 µl min−1 over 10 min using a micro-infusion device (World Precision Instruments). The needle was left in position for 20 min and slowly removed to prevent CSF regurgitation. After the FluoSpheres infusion, monkeys emerged from anaesthesia and were awake under vital monitoring. Thirty minutes after infusion of indocyanine green into the cisterna magna, lymphatics and lymph nodes in the head and neck were imaged with an infrared detector (FLUOBEAM FB800, FLUOPTICS) under isoflurane (1.5% in 2 l min−1 oxygen) anaesthesia with vital monitoring.
Intracisternal delivery of AAV9–VEGF-C
Prox1–GFP mice were anaesthetized by intraperitoneal injection of a mixture of ketamine (10 mg kg−1) and xylazine (1 mg kg−1). Five minutes before intracisternal injection, atipamezole (1 mg kg−1; A9611, Sigma) was administered intraperitoneally. One microlitre of AAV9–VEGF-C–mCherry (AAV9-275994-mCherry, Vector Biolabs) or AAV9–mCherry (7107, Vector Biolabs), with a concentration of 1 × 1013 gene copies per millilitre in PBS was infused into the SAS at the cisterna magna at 1 µl over 1 min. At 4 weeks after infusion, the nasal mucosa, hard palate, dorsal meninges and scLVs were removed for the histological analysis as described in ‘Tissue preparation and immunohistochemistry’.
Intravital imaging of scLVs
Prox1–GFP mice were anaesthetized and laid in a supine position. An imaging window for scLV-1, scLV-2 and scLV-3 was created by incising the skin overlying the lymphatics in a location 6 mm proximal and 6 mm distal to the submandibular lymph node and 5 mm from the midline to the masseter muscle. Then, the exposed lymphatics were imaged with a high-speed confocal microscope (IVIM-CM3, IVIM Engine 3.10.10, pinhole size was 2 mm, IVIM Technology) while body temperature was maintained at 37 °C with a heating pad. A GFP filter (band-pass filter for 503–558 nm) was used. After a 20–30-min period for the lymphatics to stabilize, images were acquired at 20 frames per second for 5–10 min with a Plan-Apochromat ×10/0.45 lens (Nikon). Images had a resolution of 768 × 768 pixels (0.71 μm per pixel). Lymphatics were immersed in PBS during the intravital imaging to prevent drying.
Mechanical stimulation for increasing CSF drainage
We developed a precision force-regulated mechanostimulator designed to increase CSF outflow by applying mechanical stimulation to periorbital, nasal sidewall and scLVs without surgery (Extended Data Fig. 13a). The stimulator weighing 91.3 g consisted of a replaceable tip, handle, force sensor, shaft connecting the tip and the force sensor, and amplifier. The housing of the stimulator was created with a 3D printer (Single Plus-320C, CUBICON) having a acrylonitrile butadiene styrene filament (ABS-A100, CUBICON). The replaceable tip was a 1 cm × 0.5 cm oval cotton ball attached to a 1-cm long rod that fit securely into the shaft. The length of the handle was 9 cm, preventing interference by keeping the sensor away from the hand. The force sensor made of silicone (Ecoflex 00-10, Dragonskin10, Smooth-On Inc.) and conductive fabric (Stretch conductive fabric 4800, Holland Shielding Systems) was connected to the replaceable tip through the shaft, enabling precise measurement of the force at the tip (Extended Data Fig. 13a). The measured force was transmitted to an amplifier, which relayed the data to a personal computer via a universal serial bus connector.
Transmitted data were processed with custom LabView codes (v2017, National Instruments Inc.). The device was calibrated by setting the measurements for the state when no force was applied and when a force of 0.05 kgf was applied on an electronic scale (Supplementary Video 4). The magnitude of the stimulation was optimized using this precision force-regulated stimulator. The force of mechanical stimulation was set at two levels: low magnitude (0.01–0.02 kgf) and high magnitude (0.04–0.08 kgf; Extended Data Fig. 13b).
Each 20-stroke session of mechanical stimulation lasted 1 min and consisted of a cycle of 10 downward sweeping-strokes, with each stroke lasting 2 s, followed by a second cycle of 10 strokes and then a 20-s rest period (Extended Data Fig. 13c). Three regions of intact skin were stimulated during each cycle: (1) from the periorbital area to the mandible (four strokes); (2) from the nasal sidewall to the mandible (four strokes); and (3) along the rostral to caudal path of scLV-1 and scLV-2 to the submandibular lymph node (two strokes; Extended Data Fig. 13c).
Measurement of the effects of mechanical stimulation
Prox1–GFP mice at age 8–12 weeks of age (adult) or age 80–105 weeks of age (aged) were anaesthetized exclusively by intraperitoneal injection of urethane (1.5 mg kg−1) and then received 5 or 20 1-min sessions of mechanical stimulation on one side of the face and neck. Ketamine and xylazine anaesthesia were not used because of their variable effects on CSF outflow. Corresponding sham control mice received no stimulation. Before stimulation, TMR–dextran (1 µl) was infused into the cisterna magna and then the head was immobilized with a polydimethylsiloxane head frame. After the stimulation, mice were euthanized, and TMR–dextran fluorescence in scLV-1 and scLV-2 (after 5 sessions; Fig. 6a) or the submandibular lymph node (after 20 sessions; Fig. 6d) was measured with a fluorescence stereo zoom microscope (AxioZoom V16, ZEN 2.3 (blue) v2.3.64.0, Plan-Neofluar Z ×1.0 objective lens, Carl Zeiss) and HE-GFP or Cy3 filter (Carl Zeiss).
The involvement of nitric oxide synthesis in the response to the mechanical stimulation was tested by administering the NOS inhibitor l-NAME (1.0 mg kg−1 body weight, injected intraperitoneally) 90 min before the TMR–dextran infusion at the cisterna magna of young adult Prox1–GFP mice. As previously described73, mean arterial blood pressure did not change after injection of l-NAME in mice anaesthetized with urethane. Mice pretreated with l-NAME or PBS received 20 sessions of mechanical stimulation on one side of the head and neck. Sham controls received no stimulation.
To quantify fluorescent tracers in CSF after mechanical stimulation in adult Prox1–GFP mice, TMR–dextran was first infused into the lateral ventricle, as previously described16,23, and then the facial and neck skin was stimulated. CSF was collected through the cisterna magna 30 min after the initial infusion. A small hole was drilled at the medial–lateral axis 1.5 mm and anterior–posterior axis −1.0 mm relative to the bregma after exposing the skull on a stereotaxic frame to perform intracerebroventricular infusion. A 33-gauge NanoFil needle (World Precision Instruments) connected to a PE-20 catheter was inserted to a depth of 2 mm. One microlitre of PBS containing TMR–dextran (10 kDa, 50 mg ml−1; D1816, Invitrogen) was infused into the lateral ventricle at 1 µl over 1 min using a microsyringe (88000, Hamilton) and a micro-infusion pump (Fusion 100, Chemyx Inc). After infusion, the NanoFil needle was left in place for 5 min to prevent backflow and then slowly removed. The hole was sealed with a mixture of resin and superglue. After the infusion, the mice were placed in the supine position, and mechanical stimulation was applied for 10 min to the ipsilateral side of the intracerebroventricular infusion. Ten minutes after the end of mechanical stimulation, CSF was collected through the cisterna magna. To collect CSF, the meninges were exposed using the same methods as for the cisterna magna infusion, and the 33-gauge NanoFil needle connected to a PE-20 catheter was inserted into the SAS. Using a micro-infusion pump (Fusion 200x, Chemyx Inc.), CSF was withdrawn at a rate of 1 µl min−1 for 2 min. Collected CSF was diluted in 200 µl PBS, and the fluorescence intensity of 100 µl of the mixed solution was measured with a stereo zoom microscope (AxioZoom V16, ZEN 2.3 (blue) v2.3.64.0, Carl Zeiss) with a Plan-Neofluar Z ×1.0 objective lens and Cy3 filter (Carl Zeiss). Stereo microscopic measurements of TMR–dextran in CSF were calibrated from a standard curve made from serial dilutions (Supplementary Fig. 19).
Tissue preparation and immunohistochemistry
Whole mounts of the periorbital region, nasal mucosa, hard palate, cribriform plate region and dorsal meninges of Prox1–GFP mice were prepared 20 min, 30 min, 60 min or 4 weeks after intracisternal infusion of TMR–dextran, QDot 705, FluoSpheres or AAV9-VEGF-C. The head and neck were removed at the C2 vertebral level immediately after vascular perfusion of ice-cold PBS followed by 2% paraformaldehyde (PFA) fixative through left ventricle. Blood and fixative exited through a puncture in the right atrium. Then, the mandible was removed to expose the hard and soft palate. After cutting the gingiva with a microscissor, the hard palate was removed by grabbing the soft palate with fine forceps under a surgical microscope. The hard palate was incubated in 2% PFA for 2 h at 4 °C. Then, the head was cut in half along the sagittal plane with a blade. After the overlying skin and muscle were removed, the nasal mucosa was carefully detached from the skull and lateral nasal wall, incubated in 0.5 M EDTA solution for 48 h at 4 °C, and prepared as previously described50. The periorbital area tissue was removed from the remaining half-cut head. The zygomatic arch was removed to avoid damage to associated soft tissue. The eye lens, vitreous body and retina were removed, and the soft tissue of the periorbital area was carefully separated from surrounding small bone. The nasal mucosa and periorbital area tissue were incubated in 2% PFA for 2 h at 4 °C. The dorsal meninges was processed without detachment from the skull during immunofluorescence staining. The dorsal part of skull was fixed with 2% PFA for 2 h at 4 °C and decalcified with 0.5 M EDTA solution for 12 h at 4 °C. Tissues of mice infused with QDot 705 were removed for imaging 20 min after intracisternal infusion without previous perfusion of PBS or 2% PFA.
For the whole-mount preparation of scLVs and superficial cervical lymph nodes, mice were perfused with ice-cold PBS and 2% PFA, and cervical lymphatics and lymph nodes were removed with surrounding tissue, pinned with insect pins to prevent tangling, and post-fixed in 2% PFA for 2 h at 4 °C. The tissues were washed with PBS and incubated in CUBIC-L solution (T3740, TCI) with daily change for 3 days at 37 °C. After clearing and PBS washing, tissues were stained for immunofluorescence and imaging.
Monkeys were perfused through the vasculature with ice-cold PBS and 4% PFA. The submandibular, parotid and retropharyngeal lymph nodes were removed, further fixed with 2% PFA for 12 h at 4 °C, dehydrated with 30% sucrose at 4 °C, and washed in PBS. After 48 h, lymph nodes were embedded and frozen in frozen section medium (Leica) and cut into 10-μm sections using a Cryocut Microtome (Leica). The head specimens were fixed with 4% PFA for 2 h and 2% PFA for 24 h at 4 °C. The lower mandibles were removed and the hard palate was collected. The hard palate was imaged using a fluorescence stereo zoom microscope (AxioZoom V16, ZEN 2.3 (blue) v2.3.64.0, Carl Zeiss) with a Plan-Neofluar Z ×1.0 objective lens with HE-GFP or Cy3 filter (Carl Zeiss).
Tissues from mice and monkeys were incubated in 5% normal donkey serum (017-000-121, Jackson ImmunoResearch) for 1 h at room temperature and then incubated with primary antibodies (1:400) dissolved in 5% normal donkey serum at 4 °C for 12 h. After washing in PBS, they were incubated with secondary antibodies (1:1,000) dissolved in 5% normal donkey serum at 4 °C for 12 h. Specimens that had been cleared were incubated with donkey serum for 24 h at room temperature and fluorophore-conjugated primary antibodies at 1:200 dilution at room temperature for 5 days. After PBS washing, the specimens were immersed in a refractive index matching solution (D-PROTOSS)74.
Primary antibodies used were: anti-mouse LYVE1 (rabbit polyclonal; 11-034, Angiobio); anti-mouse VEGFR3 (goat polyclonal; AF743, R&D Systems); anti-mouse αSMA-Cy3 (mouse monoclonal, clone 1A4; C6198, Sigma); anti-mouse laminin α5 (rabbit polyclonal; EWL004, kerafast); anti-mouse CD31 (hamster monoclonal, clone 2H8; MAB1398Z, Merck); anti-mouse tyrosine hydroxylase (rabbit polyclonal; AB152, Merck); anti-mouse vesicular acetylcholine transporter (VAChT, also known as solute carrier family 18 (vesicular acetylcholine), member 3, Slc18a3, goat polyclonal; ABN100, Merck); anti-monkey LYVE1 (rabbit polyclonal; DP3500, OriGene); anti-mouse eNOS antibody (rabbit polyclonal; ab5589, Abcam); anti-mouse phospho-eNOS antibody (rabbit polyclonal; 9571, Cell Signaling); anti-mouse FOXP2 antibody (goat polyclonal; ab1307, Abcam); anti-mouse ER-TR7 antibody (rat monoclonal, clone ER-TR7; sc-73355, Santa Cruz Biotechnology); anti-mouse Col1a1 (rabbit monoclonal, clone E8F4L; 72026, Cell Signaling); and anti-mouse PDGFRα (goat polyclonal; AF1062, R&D Systems). Secondary antibodies were: Alexa Fluor 488-conjugated, 594-conjugated and 647-conjugated anti-rabbit (711-545-152, 711-585-152 and 711-605-152, respectively), anti-goat (705-585-147), anti-hamster (127-605-160) and anti-rat (712-605-153; all Jackson ImmunoResearch) in the blocking buffer for overnight at 4 °C. The manufacturers validated the species and applications of all the antibodies used in this study.
RNA in situ hybridization
RNA in situ hybridization for Nos3 and Postn mRNA in scLV whole mounts from Prox1–GFP mice was performed using the RNAscope kit (323280, ACDBio) with target probes to Nos3 (443061-C2, ACDBio) and Postn (418581-C2, ACDBio), according to the manufacturer’s specifications. scLVs were fixed with 2% PFA overnight at 4 °C and then permeabilized by incubation in a mixture of 0.1% Tween 20 in PBS and 1% bovine serum albumin (BSA). Target retrieval was omitted to preserve the Prox1–GFP signal. The samples were imaged and analysed as described in ‘Imaging and morphometric analysis’.
Imaging and morphometric analysis
Immunofluorescent images were acquired with an LSM800 or LSM880 confocal microscope (Carl Zeiss). ZEN 2.3 (blue) v2.3.69.1010 and ZEN 2.1 SP3 (black) v14.0.4.201 software (Carl Zeiss) was used for image acquisition and processing. Confocal images of tissue whole mounts and sections were projected at maximum intensity of tiled or single z-stack images through the entire thickness of tissues. All images had a resolution of 512 × 512 or 1,024 × 1,024 pixels and were obtained with an air objectives Plan-Apochromat ×10/0.45 numerical aperture (NA) M27, Plan-Apochromat 20x/0.8 NA M27 or a water immersion objective LD C-Apochromat ×40/1.1 NA Corr M27 with multichannel scanning in the frame. Specimens that underwent tissue clearing and decalcification were imaged with a light-sheet fluorescence microscope (Lighsheet 7, Carl Zeiss) with an EC Plan-Neofluar ×5/0.16 lens.
Morphometric measurements were made with ImageJ software (NIH) or Zen software (Carl Zeiss) on maximum-intensity-projected confocal images. VEGFR3+ lymphatic area, LYVE1 signal intensity, lymphatic vessel diameter and number of lymphatic valves were measured on the submucosal side of the hard palate at the two ROIs: ROI-1 (greater palatine nerve area, 850 μm × 650 μm), ROI-2 (near the CSF outflow area, 450 μm vs 450 μm). The VEGFR3+ area in ROI-1 and ROI-2 was measured using the Weka trainable segmentation of ImageJ plugin75. The diameter of lymphatics and number of lymphatic valves were counted with ImageJ. Signal intensities of LYVE1 were measured in the submucosal side of the hard palate in the region (ROI-1 and ROI-2) defined by the aforementioned boundaries. The VEGFR3+ lymphatic area and lymphatic vessel diameter in the nasal mucosa were measured in ROI-1 and ROI-2 measuring 330 μm × 330 μm. In specimens from Prox1–GFP mice, the area of GFP fluorescence of lymphatics and lymphatic diameter were measured in meningeal lymphatics on the transverse sinus and extracranial lymphatics in the nasal mucosa and submucosal side of the hard palate using the Weka trainable segmentation of ImageJ plugin75. The diameter of Prox1–GFP lymphatics was measured with ImageJ. Measurements were made at ROIs: ROI-1 (respiratory mucosa, 700 μm × 700 μm), ROI-2 (inferior nasal mucosa, 700 μm × 700 μm) and ROI-3 (submucosal side of the hard palate near the incisive foramen, 450 μm × 450 μm). The diameter, number of lymphatic valves and length of lymphangions in scLVs were measured with ImageJ. αSMA+ smooth muscle coverage per lymphangion was measured in at least three lymphangions of each scLVs using the Weka trainable segmentation of the ImageJ plugin75. The Nos3 and Postn mRNA signal was measured with ImageJ by counting the number of spots in random regions (159 μm × 159 μm) that included lymphangions of scLV. Type 1 collagen expression was measured by average fluorescence intensity in random regions (830 μm × 830 μm) that included the scLV and facial vein.
Intravital images were aligned with an Image Stabilizer of the ImageJ plugin76. Then, a perpendicular line was drawn to obtain intensity profiles for 1–6 min using a custom ImageJ macro. For each intensity profile, the full-width half-maximum was extracted to measure the diameter of scLVs with a custom MATLAB (R2023b, MathWorks) code. The diameter of these lymphatics was used to calculate the amplitude of spontaneous contraction and relaxation, frequency, ejection fraction and fractional pump flow for 1 min as previously described37. To determine whether spontaneous contractions and relaxations were synchronized within each lymphangion, cross-correlations of diameter changes were calculated at five locations, spaced 40 μm apart, using a custom MATLAB code.
Ex vivo studies of pressurized scLVs
Mice were anaesthetized by intraperitoneal injection of a ketamine and xylazine mixture and laid in a supine position on a heated pad. A proximal-to-distal incision was made in the skin from the neck to the sternum. A 0.5–1.5-mm long segment of scLVs on both sides was removed with fine forceps and microscissors and transferred to a dish containing Krebs solution with 0.5% BSA. The lymphatics were then pinned with short segments of 40-µm stainless steel wire onto a Sylgard-coated dissection chamber filled with Krebs-BSA buffer at room temperature. Surrounding adipose and connective tissues were removed by microdissection. An isolated lymphatic was then transferred to a 3-ml observation chamber on the stage of a Zeiss inverted microscope, cannulated, and pressurized to 1 cmH2O using two glass micropipettes (50–60 µm outside diameter). With the vessel pressurized, the segment was cleared of remaining connective and adipose tissue.
Polyethylene tubing was attached to the back of each glass micropipette and connected to a computerized pressure controller, with independent control of inflow and outflow pressures. To minimize diameter-tracking artefacts associated with longitudinal bowing at higher intraluminal pressures, input and output pressures were briefly increased to 10 cmH2O at the beginning of each experiment, and the vessel segment was stretched axially to remove any longitudinal slack. Following this procedure, each lymphatic was equilibrated at 37 °C at a pressure of 1 cmH2O. Constant exchange of Krebs buffer was maintained at a peristaltic pump rate of 0.5 ml min−1. Within 30 min after the temperature stabilized, scLVs began to exhibit spontaneous contractions. Custom LabView programs (National Instruments Inc.) acquired real-time analogue data and digital video through an A-D interface (National Instruments Inc.) and detected the inner diameter of the vessel37. Videos of the contractile activity of lymphatics were recorded for further analyses under brightfield illumination at 30 fps using a firewire camera (Basler, Graftek Imaging).
Ex vivo assessment of lymphatic responses
To assess physiological responses of scLV segments to pressure changes, intraluminal pressure was lowered from 1 to 0.5 cmH2O, then raised to 1, 2, 3, 5, 8 and 10 cmH2O, while the internal diameter was recorded for 1–2 min at each pressure. Both the input and the output pressures were maintained at equal levels, so there was no imposed pressure gradient for forwards flow. After the pressure was returned to 1 cmH2O for 5 min, phenylephrine was added to the bath in cumulative concentrations, while the diameter was recorded for 1–2 min at each concentration. Once a maximum tone was reached (typically 40–50% of the passive diameter), diethylamine-NONOate sodium salt hydrate (sodium NONOate, Merck) was applied in cumulative concentrations, and the diameter was measured at each concentration. At the end of each experiment, the lymphatic was equilibrated by perfusion with calcium-free Krebs buffer containing 3 mM EGTA for 30 min, and passive diameters were measured at each intraluminal pressure.
Droplet-based scRNA-seq
After anaesthesia, Prox1–GFP adult mice (n = 3 males and n = 3 females at 12 weeks of age) and aged mice (n = 3 males and n = 3 females at 93 weeks of age) were perfused with ice-cold PBS. scLVs near the submandibular lymph node and surrounding tissue were removed and pooled in DMEM/F12 medium (Gibco). The tissues were cut into small pieces and incubated in dissociation buffer containing 2 mg ml−1 of collagenase II (Worthington), 1 mg ml−1 of dispase (Gibco) and 0.2 mg ml−1 DNase I (Gibco) at 37 °C for 30 min with gentle pipetting up and down every 10 min. Dissociated tissue was then filtered through a 70-µm strainer, and an equal volume of PBS containing 10% FBS was added to stop digestion. The cells were centrifuged for 10 min at 300g and resuspended in PBS containing 2% FBS. The resuspended cells were incubated with anti-PDGFRα antibody-conjugated Microbead Kit (103-101-502, Miltenyi Biotec) on ice for 15 min to separate PDGFRα+ cells from PDGFRα− cells using magnetic columns (130-042-401, Miltenyi Biotec). Then, immune cells and dead cells were removed by FACS (FACSAria Fusion, Beckton Dickinson) after staining with anti-mouse CD45 antibody (103147, clone 30-F11, BioLegend) and DAPI (564907, Sigma-Aldrich) at 4 °C for 20 min. Subsequently, PDGFRα+ cells and PDGFRα− cells were mixed in a 2:8 ratio to enrich PDGFRα− cells population to more than 15%.
The cells were then centrifuged for 10 min at 300g, resuspended in 2% FBS in PBS, and processed using Chromium Next GEM Single Cell 3p RNA library kit v3.1 (10X Genomics) following the manufacturer’s protocol. The cells were mixed with reverse transcription reagent mix and reverse transcription primer, loaded to 10X chips and separated into gel beads in emulsion, where transcripts from individual cells were uniquely barcoded. Barcoded transcripts were reverse transcribed and amplified to generate cDNA libraries. Size selection of the initial cDNA libraries was performed using SPRI beads (Beckman Coulter). After fragmentation and adaptor ligation, libraries were amplified with sample index primers. Following another round of double-sided size selection, the qualities of the final libraries were checked by Agilent Bioanalyzer High Sensitivity Chip. The Illumina Novaseq6000 platform sequenced libraries that passed the final quality control.
Pre-processing of scRNA-seq data
For droplet-based scRNA-seq, sequenced libraries were demultiplexed and aligned to the mouse reference genome GRCm39 (mm10), with the eGFP sequence added to the reference, using Cell Ranger (v8.0.1). Raw expression matrices were constructed using Read10X function in the ‘R’ package Seurat (v5.0.3). Before clustering analysis, low-quality cells detected with fewer than 1,000 genes and putative dead cells with high mitochondrial percentage (more than 10% of reads mapped to mitochondrial genes) were discarded. Cells detected with more than 6,000 genes were also considered doublets and removed. For gene-based quality control, genes detected in fewer than three cells were removed from the raw expression matrix. After quality control of unwanted cells and genes, expression matrices were normalized column-wise, dividing the unique molecular identifier (UMI) counts of each gene by the sum of UMI counts for a given cell. Then, scale factor 10,000 was multiplied, and log2 transformed to yield log counts per million equivalent values.
Clustering analysis
Clustering and downstream analyses were performed by the ‘R’ package Seurat (v5.0.3). First, the 2,000 most variable genes for each dataset were identified by the FindVariableFeatures function with options: selection.method = ‘vst’. For initial dimensionality reduction, principal component analysis was performed. The top 20 principal components were selected for further downstream analysis, such as uniform manifold approximation and projection for two-dimensional visualization and shared nearest neighbour graph for neighbour detection and cluster identification using the Louvain algorithm. After initial clustering, the small number of contaminating cells and doublet cells were excluded. After removal of the cells, the remaining cells were dimensionally reduced and clustered again, as described above. For the identification of differentially expressed genes across clusters, the FindMarkers function in Seurat was used with the following options: test.use = ‘MAST’, min.pct = 0.3 and logfc.threshold = 0.3. In merging adult and aged mouse datasets, no batch correction method was used, as no batch effect was observed for clustering.
Statistical analysis
Sample sizes were chosen on the basis of standard power calculations (with α = 0.05 and power of 0.8) and no statistical methods were used to predetermine sample size. Experiments were randomized, and investigators were blinded to allocation during experiments and outcome assessment. Data were tested for normality using the Shapiro–Wilk and Kolmogorov–Smirnov one-sample test. Parametric or non-parametric statistical tests were used, depending on the data distribution. The statistical significance of differences was determined by two-tailed Student’s t-test, two-tailed Welch’s t-test, Brown–Forsythe ANOVA test, two-way ANOVA test, two-way repeated measure ANOVA test, Kruskal–Wallis test, or two-tailed Mann–Whitney U-test, two-tailed Tukey’s multiple comparison test, two-tailed Sidak’s multiple comparison test, two-tailed Dunn’s multiple comparison test, two-tailed Dunnett’s T3 comparison test, two-tailed model-based analysis of single cell transcriptomics with Bonferroni post-hoc test, and one-tailed Fisher’s exact test with false discovery rate correction using the Benjamini–Hochberg method. Statistical analyses were performed using Prism 10 (GraphPad Software, v10.2.3). All data are expressed as mean ± s.e.m. Statistical significance was set at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.