Mosquito rearing
A. gambiae G3 mosquitoes were housed in cages in an insectary maintained at 27 °C with 70–80% humidity and a 12-h light–dark cycle. Colony cages were provided with water, 10% w/v glucose solution ad libitum and donated human blood (Research Blood Components) once a week for colony maintenance. For the experiments described in this article, G3 pupae were collected and placed into cages for eclosion, after which adult mosquitoes were provided with water and a 10% w/v glucose solution until the time of experimentation. All experiments were performed with age-matched female mosquitoes ranging from 4 to 7 days old.
P.
falciparum culture and gametocyte induction
Wild-type NF54 P. falciparum (used in accordance with a material-transfer agreement from the laboratory of C. Barillas-Mury) was used for all experiments in this article, with the exception of the following experiments: (1) the Dd2 CytB I22L and TM90-C2B CytB Y268S mutant lines (both courtesy of the laboratory of M. Riscoe) used for cross-resistance analysis; and (2) the CytB V259L, V284L and M133I resistant NF54 lines generated through ELQ selection. Wild-type and mutant NF54 cell lines were authenticated by whole-genome sequencing. Dd2 I22L was authenticated by Sanger sequencing33, and TM90-C2B is a clinical isolate34. All cell lines were tested for mycoplasma contamination, and TM90-C2B was mycoplasma-positive (all others were negative). Asexual blood-stage parasites were maintained between 0.2 and 2% parasitaemia in RPMI 1640 with l-glutamine and 25 mM HEPES medium (Corning) supplemented with 10 mg l–1 hypoxanthine, 0.2% sodium bicarbonate and 10% heat-inactivated human serum (Interstate Blood Bank) and 5% haematocrit in human red blood cells (Research Blood Components) in incubators kept at 37 °C and a gas composition of 5% O2, 5% CO2 and balanced N2 for up to 2 months per established protocols49. Gametocytes were induced by increasing the parasitaemia to >2% and incubating for 14–20 days with daily medium changes to accumulate stage V gametocytes per established protocols50.
A.
gambiae infection with P.
falciparum
A. gambiae G3 mosquitoes (aged 4–7 days) were fed on 14–21-day P. falciparum NF54 induction cultures containing stage V gametocytes kept at 37 °C in custom glass, water-jacketed membrane feeders. Mosquitoes were allowed to feed for up to 60 min and then were placed into a custom-made infection glovebox for biosafety containment (Inert Technology). Any mosquitoes that did not fully engorge were aspirated into 80% ethanol and removed from subsequent experimentation. Mosquitoes were provided with water and a 10% w/v sugar solution to feed ad libitum for the duration of the experiment, and the room was kept at 27 °C with 70–80% humidity on a 12-h light–dark cycle.
Topical applications
DMSO stocks (100 mM) were prepared for each compound in the topical-application screening library and stored in individual aliquots at −20 °C. On the day of topical application, DMSO stocks were thawed and diluted 1:50 in acetone to make the final 2 mM and 2% v/v DMSO working solution. A 2% DMSO control solution was also prepared in this manner. We chose this screening concentration to maximize the amount of compound applied to mosquitoes while minimizing the DMSO concentration. For compounds that were insoluble at 100 mM DMSO, 2 mM working solutions were instead prepared directly from powder stock with 2% v/v DMSO and acetone. In rare cases (noted in Supplementary Table 1), for compounds that were insoluble at 100 mM DMSO, working solutions were instead prepared from a saturated DMSO stock to maximize the final screening concentration (at a maximum solubility limit of less than 2 mM). Female A. gambiae G3 mosquitoes (aged 4–7 days) were anaesthetized on ice and 0.5 µl working solution or DMSO control was pipetted directly onto the dorsal thorax. Mosquitoes were removed from the ice and placed into a cage to recover for 4–6 h before being provided an infectious blood meal. All compounds were screened once, and compounds that showed a preliminary reduction in parasite prevalence were assayed at least once more in a second biological replicate to confirm activity.
Tarsal-contact assays
Tarsal-contact assays were performed as previously described3, but with some modifications. Tarsal-contact plates were prepared by calculating the amount of compound necessary to coat a 6-cm-diameter glass Petri dish (0.02827 m2 total surface area) at 1 mmol m–2. This concentration is roughly 10× the previously described atovaquone tarsal-contact EC99 value9, and we reasoned that this high concentration would enable detection of active compounds. Powder compounds were weighed out and solubilized in acetone and diluted as necessary to reach the calculated surface-area concentration. Next, 100 mg m–2 of the adjuvant Mero (also known as rapeseed methyl esters and emulsifier ethoxy (7) tridecanol (RME), a gift from H. Ranson) was also added to each solution, which has previously been shown to act as an uptake excipient in mosquito tarsal-contact assays51. For ELQ-453–ELQ-613 combination exposure, solutions were prepared so that the total compound concentration was 1 mmol m–2 made up of 0.5 mmol m–2 ELQ-453 and 0.5 mmol m–2 ELQ-613. Stock solutions were serially diluted to prepare lower concentration plates for tarsal contact dose–response curves. The final 1 ml acetone solution containing 100 mg m–2 RME and the compound at the desired concentration was pipetted onto a 6 cm glass Petri dish. A 100 mg m–2 RME control plate was also prepared. The plates were left at room temperature overnight on an orbital shaker to fully evaporate the acetone. The next day, plates were fitted with a transparent plastic lid to contain mosquitoes during exposure. Female A. gambiae G3 mosquitoes (4–7 days old) were mouth aspirated into the exposure apparatus for 6 min. A maximum of 25 mosquitoes were exposed at a time to minimize crowding and to allow all mosquitoes to come into contact with the drug-coated or vehicle-coated surface. In each experiment, mosquitoes were visually monitored to confirm tarsal contact with the coated surface. We did not observe obvious excessive flight or other apparent indications of avoidance behaviour, although this was not empirically recorded. After 6 min, mosquitoes were released into a cage and provided with an infectious blood meal 1 h later. For post-infection tarsal contact, exposures were performed as previously described4. At 3 days after an infectious blood meal, mosquitoes were transferred into the exposure apparatus as described above, and after 6 min of tarsal-contact exposure, they were transferred to a new cage by mouth aspiration. For thin-film and dipped-net tarsal contact, exposures were performed as described above with 6-cm-diameter plastic lids fitted to the polyethylene thin films or the dipped net to contain mosquitoes.
Net-dipping protocol
White polyester netting (12.5 × 12.5 cm) was used for tarsal-contact assays on nets. The netting was initially soaked in acetone and then allowed to air dry for 30 min. After this time, netting was soaked in equivalent amounts of ELQ-453 and ELQ-613 in acetone or in acetone alone (control) to achieve the desired ELQ mg m–2 concentration and rocked until the entire solution was absorbed. Netting was allowed to air dry overnight on a flat surface, and tarsal-contact assays were performed the next day.
Oocyst dissection and quantification
For all oocyst data, at 7 d.p.i., mosquitoes were aspirated into 80% ethanol, incubated at –20 °C for 10 min, then transferred to 1× PBS at room temperature for dissection. Midguts were dissected into 1× PBS, stained by transferring into 0.2% w/v mercury dibromofluorescein disodium salt solution (mercurochrome, Sigma-Aldrich) in ddH2O for 14 min and mounted in 0.02% w/v mercurochrome for microscopy. Midguts were imaged and oocysts counted at ×100 magnification on an Olympus Inverted CKX41 microscope. For post-infection experiments, oocyst size (cross-sectional area) was measured using the ImageJ software fork Fiji52.
Sporozoite dissection and quantification
At 11 or 14 d.p.i., mosquitoes were aspirated from their cages in the infection glovebox into 80% ethanol, incubated at −20 °C for 10 min, then transferred to 1× PBS at room temperature for dissection. Mosquito heads were removed and salivary glands from individual mosquitoes were dissected and collected in 50 µl 1× PBS. Salivary gland tissue was mechanically disrupted with a small handheld pestle and 10 µl was loaded onto a haemocytometer. Sporozoites were counted using an Olympus Inverted CKX41 microscope at ×100 magnification with phase contrast, and the total number of sporozoites per mosquito was calculated.
Live ookinete IFAs
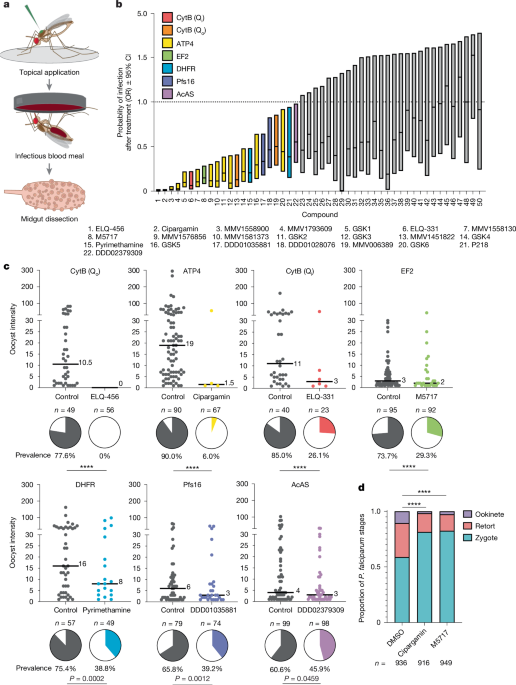
Cipargamin, M5717, ELQ-613 or 2% DMSO as control was topically applied to mosquitoes before an infectious blood meal as described above. At 22 h.p.i., 10 mosquitoes per group were aspirated into 80% ethanol, incubated at −20 °C for 10 min, then transferred to 1× PBS at room temperature for dissection. Midguts were dissected with their blood bolus intact into 1.5 ml low-bind Eppendorf tubes containing 100 µl 1× PBS. The midgut tissue was disrupted by gently pipetting midguts up and down approximately 20 times until the blood bolus was visibly released and midguts were no longer intact. This homogenate was centrifuged at 300g for 5 min and the supernatant was discarded. The pellet containing homogenized blood and midgut tissue was resuspended in 100 µl of 1× PBS with 1:300 mouse anti-PfS25 (BEI Resources) primary antibody conjugated to CF-488 dye (Sigma-Aldrich) and 1:500 Hoechst (AAT Bioquest) and incubated in the dark at −4 °C for 30 min. After incubation, samples were again spun down at 300g for 5 min and resuspended in 100 µl 1× PBS wash. Samples were spun down again and resuspended in a final volume of 10 µl 1× PBS, which was mounted onto a microscope slide (VWR) and imaged on an inverted Zeiss Axio Observer Z1 at ×200 magnification. Approximately 200 parasites per group were live counted and classified as a zygote, an intermediate retort form or an ookinete on the basis of parasite morphology.
Sample preparation for MS analysis
Female mosquitoes were exposed to test compounds (100 µmol m–2 ELQ-453, ELQ-456, ELQ-458, ELQ-613, ELQ-614, ELQ-618 and ELQ-465) through 6 min of tarsal contact as described above, transferred to new cages and provided with a continuous supply of water and 10% w/v glucose solution until the end of the experiment. Midguts from treated mosquitoes were then collected at the following time points after tarsal-contact exposure: 1, 3, 6, 24 and 48 h. Ten mosquitoes per time point were aspirated into 80% ethanol, transferred into PBS on ice and dissected. For each time point, 5 whole midguts were collected and pooled into 1.5 ml Eppendorf tubes containing 400 μl methanol and a 2 mm glass bead, resulting in 2 technical replicates per time point. Two biological replicates (with two technical replicates each) were performed in total. Samples were stored at −80 °C until homogenization.
ELQs with similar structures to the test compounds were used as internal standard (IS) samples for detection and quantification: prodrug ELQ-615 and its active form ELQ-183, and prodrug ELQ-331 and its active form ELQ-300. An IS master mix was prepared with 2 nM of each standard in methanol. Next, 100 μl of this IS master mix was added to each sample to obtain a total final volume of 500 μl. Samples were homogenized through bead beating in a cold block at 2,400 r.p.m. for 1.5 min, 3 times. After homogenization, the samples were centrifuged for 10 min at 15,060 r.p.m. at 4 °C. The supernatant, containing the extracted material, was carefully transferred to new 1.5 ml Eppendorf tubes. Samples were kept on ice throughout the extraction process. The prepared supernatants were stored at −80 °C until submission to the Harvard Center for MS analyses.
MS analysis
All solvents were LC–MS grade (Millipore Sigma). Samples were evaporated to dryness under nitrogen flow and resuspended in 20 µl methanol 80% in water. Samples were quantified using an ABSciex 7500 triple quadrupole mass spectrometer (AB Sciex) coupled to an 1290 Agilent LC (Agilent). Five microlitres of the sample was injected into a Kinetex C18 column (2.1 mm × 150 mm, Phenomenex) maintained at 55 °C. The mobile phases were A (water and 0.1% formic acid) and B (acetonitrile and 0.1% formic acid). The following gradient was used: 10% B for 5 min, then to 100% B in 13 min, maintained at 100% B for 4 min, followed by 2 min re-equilibration at 10% B. The flow rate was 0.3 ml min−1. The instrument was operated in positive mode with the following source conditions: gas 1 at 40 psi, gas 2 at 65 psi, curtain gas at 44, collision (CAD) gas at 8, temperature at 525 °C and spray voltage at 1,800 V. See Supplementary Table 3 for the multiple reaction monitoring transitions used. A shorter cleaning method was used to inject a solvent blank between each sample. For the standard curve, a series of solutions in methanol were prepared, with the highest at 500 nM for all targets and 12 successive one-third dilutions. One hundred microlitres of each solution was added to unexposed midgut homogenates. The standard curve samples were then processed as for the samples. Targets were quantified using the area under the curve for the quantifier transitions divided by the area of the quantifier of the corresponding internal standards using Sciex OS Analytics (AB Sciex).
Asexual blood-stage parasite culture for resistance selection
NF54 parasite lines (obtained through a material-transfer agreement from the laboratory of C. Barillas-Mury) were grown at 4–5% haematocrit in fresh human erythrocytes (O+) in RPMI 1640 medium supplemented with 10% O+ human serum (heat-inactivated and pooled), 26.6 mM NaHCO3, 27.7 mM HEPES, 0.41 mM hypoxanthine and 25 µg ml–1 gentamicin. Human serum and erythrocytes were supplied by Interstate Blood Bank. Cultures were incubated at 37 °C under 1% O2, 5% CO2 and balance N2 gas.
Selection of CytB mutants
To generate CytB mutants, NF54 parasites were exposed to various CytB inhibitors as follows. Parasites were exposed to 2 nM atovaquone (M133I), 5 nM or 10 nM ELQ-121 (V259L), 5 nM or 10 nM ELQ-400 (V259L and V284L, respectively), or 2 nM or 10 nM for ELQ-437 (V259L and Y126C mixed) and kept under constant drug pressure until parasites recurred. For ELQ-300, parasites were incubated with 50 nM ELQ-300 (H12Q) for 5 weeks, after which drug pressure was removed to let parasites recover. Mutations were identified by amplification of the CytB-encoding gene (MT-CYB) by PCR (primers: MT-CYB 5′ UTR: GGATGGAATATGATTTGTTCTATTGGG and mitoR: TTATATGTTTGCTTGGGAGC) and subsequent Sanger sequencing, except for the H12Q MT-CYB Qi-site mutation, which was identified by whole-genome sequencing as described below.
Whole-genome sequencing of CytB mutants
Parasite samples from parental-susceptible NF54 and selected mutants were collected from asexual blood-stage cultures and from gametocyte cultures directly before infectious blood meals. Parasite DNA was extracted using DNeasy Blood & Tissue kits (Qiagen) for whole-genome sequencing. Sequencing libraries were prepared by the University of California San Diego Institute for Genomic Medicine Genomics Center using a Nextera XT kit (FC-131-1024, Illumina) with 2 ng input gDNA and standard dual indexing.
Raw sequencing reads were aligned to the P. falciparum 3D7 reference genome53 (PlasmoDB v.13.0) and preprocessed following standard GATK (v.3.5) protocols. GATK HaplotypeCaller was used to call single-nucleotide variants and insertions and deletions, which were hard filtered based on the basis of the following exclusion criteria: ReadPosRankSum >10.0 or <–10.0; quality by depth < 1.5; or mapping quality rank sum (MQRankSum) <–14. Variants were annotated using SnpEff with a custom database built from the 3D7 reference GFF from PlasmoDB (v.13.0). Fisher’s exact test comparing reference versus alternate allele counts in each sample to its parent was used to compute a P value for each variant call to assist in determining whether it significantly differed from the drug-sensitive parent at the same genomic locus.
Copy number variations (CNVs) were detected from whole-genome sequencing data by calculating denoised log2 copy ratios across gene intervals using the GATK 4.0 CNV pipeline. Read counts for each sample were computed across a predefined gene interval list with intergenic regions and the highly variable antigenic var, rifin, stevor and surfin genes were removed. Denoising was performed against a panel of normals consisting of 30 independently sequenced 3D7 parent clones (excluding parental lines in this study). CNVs were retained if at least 4 sequential genes showed a denoised log2 copy ratio of at least 0.6, which indicated gene amplification, or at most −0.6, which indicated a deletion. ELQ121-NF54-R-ABS was excluded from CNV analysis owing to extreme variability in copy ratios across the genome. No CNVs in core regions were found using this method.
In vitro drug susceptibility assays by SYBR Green I staining
Drug susceptibility assays were performed as previously described using the SYBR Green I method54. In brief, synchronized rings at 1% parasitaemia and 1% haematocrit in 40 µl of 0.5% serum-complimented medium were grown for 72 h in 384-well clear bottom plates in the presence of different drug concentrations. All drug conditions were performed in three technical replicates, with at least three biological replicates. Drugs were dispensed in 12-point dilution series into 384-well plates by aa HP D300e Digital Dispenser (Hewlett Packard). Growth at 72 h was quantified by staining parasite DNA with SYBR Green I (Lonza) for 24 h and measuring relative fluorescence units at an excitation of 494 nm and an emission of 530 nm using a SpectraMax M5 (Molecular Devices). EC50 values were calculated using a nonlinear regression with the log(inhibitor) versus response–variable slope curve-fitting algorithm in GraphPad Prism (v.10; GraphPad Software).
Statistics
All statistical analyses were performed in GraphPad Prism (v.10.0; GraphPad Software) and JMP Pro 17 (SAS Institute). Experimental sample sizes, randomization and blinding were based on previously published protocols3,4 and are described in detail in the Reporting Summary. For topical screening and secondary tarsal screening, the control versus compound-treated infection prevalence two-sided OR and 95% CI values were calculated using the Baptista–Pike method. The OR and 95% CI were plotted, and any treatment group with an upper confidence interval of <1 was considered a significant reduction in infection prevalence. No compounds were found to significantly increase infection prevalence. In the case of two compounds (DSM161 and DSM703), both the control and compound-treated group had 100% prevalence. In this case, two out of the four cells in the contingency table were 0 and the OR could not be calculated. We therefore arbitrarily added 1 to the uninfected cells for each group to calculate and plot an estimated OR. We similarly added 1 to the uninfected MMV019881 contingency table cell, as otherwise the calculated OR upper CI was infinity. For infection experiments, prevalence was analysed using two-tailed Fisher’s exact tests, and intensity was analysed using two-tailed Mann–Whitney tests. In experiments for which more than two groups were compared, prevalence was analysed using Fisher’s exact tests followed by Bonferroni correction, and intensity was analysed using Kruskal–Wallis tests with Dunn’s post hoc multiple-comparisons correction. For 22 h.p.i. IFAs, the numbers of each stage of parasite development (1, zygote; 2, retort; 3, ookinete) detected in midguts (1 pool of 10 midguts; 5 biological replicates) of treated mosquitoes at 20–22 h.p.i. were analysed using an ordinal logistic regression model in JMP Pro 17 (SAS Institute), incorporating ‘treatment’ (control (DMSO), cipargamin, M5717 or ELQ-613) and ‘mosquito sample’ (nested within treatment).
QSAR analysis of topical activity
An initial set of 38 compounds was used for QSAR modelling (Supplementary Table 2). This set included compounds that shared the mechanism of action with the selected hits in the topical screen and atovaquone, which showed full activity (topical OR = 0). The OR topical mean value (dependent variable) was transformed according to the following formula: 1 – [log(OR topical) + 1)]. This formula allows activity values to fall between 0 (lowest activity) and 1 (highest activity). The molecular operating environment (MOE) software (v.2022.02; Chemical Computing Group) was used to prepare the library, calculate descriptors and run the QSAR analysis. In MOE, molecules were washed to their dominant ionization form at pH 7 and energy minimization was applied to generate the three-dimensional structure from the two-dimensional canonical SMILES. The compound collection was subdivided into 27 chemical clusters (85% similarity) using MACCS fingerprint. Based on their activity values and chemical clusters, molecules were evenly distributed into training set (85%, 32 compounds) and test set (15%, 6 compounds) (Supplementary Table 2). A total of 347 molecular descriptors (209 i2D descriptors and 138 i3D descriptors) were computed. Descriptors that correlated better with the dependent variable were selected using the contingency module (196 descriptors). Three model types were built using the AutoQSAR and QSAR-Evolution codes of MOE: partial least dquare (PLS), principal component regression (PCR) and genetic algorithm–multiple linear regression (GA-MLR). To prevent model overfitting during model generation, low variance (threshold 0.9) descriptors were excluded, and the number of descriptors in the model was limited to six. Outliers (z′ score limit of 2.5) were excluded during model building. Predictive precision and goodness-of-fit of each model was assessed using the coefficient of determination (R2) and root mean square error (RMSE). To evaluate overfitting, cross-validation was run using the leave-one-out cross-validation (LOO-CV) method, giving \({R}_{{\rm{CV}}}^{2}\) and RMSECV. External validation on the test set was assessed with correlation coefficient \({R}_{{\rm{EXT}}}^{2}\). The best model obtained for topical activity was model GA-MLR, whereas PLS and PCR had poor fitting and did not satisfy the threshold external validation parameters. Correlation plot graphs comparing observed and predicted values were plotted using GraphPad Prism (v.10.0; GraphPad Software).
Compound library evaluation through CACTI
SMILES for each compound tested in this study were saved as a tabular file and queried using CACTI55 command line version and Python 3.5 (Supplementary Table 4). The search was performed to obtain analogues (flag -simnet -sdb) with 90% similarity threshold, as well as literature references (flag -lit -slit) for both the compound library and analogues. Chemical names and SciFinder CAS identifiers (numerical series) found by CACTI were extracted from the results of synonyms and inputted as an additional column for easier access. Compounds with missing entries were obtained using ChemDraw 22.2 and the SciFinder CAS-n web tool when available. Public references obtained with CACTI were manually confirmed, and cases in which CACTI found evidence of a target different from our study are indicated. Analogues with a validated target were used for validation and the rest filtered to reduce the complexity of the CACTI analysis.
ELQ synthesis
Synthetic methods and small-molecule characterization are provided in the Supplementary Information and Supplementary Table 5.
Preparation of ELQ LDPE films
LDPE polymer (0.5 g) and 25 ml xylenes were combined in the lid of a 6 cm glass Petri dish and heated at 150 °C until the polymer dissolved. It was necessary to occasionally replenish evaporated xylenes. ELQ in xylenes was then added to this solution. Excess xylenes were evaporated slowly and the resulting crude polymer films were allowed to dry overnight. The bottom of the 6 cm glass Petri dish was then placed against the crude polymer thin film. The assembled apparatus was then heated on a hotplate with a weight placed against the bottom of the Petri dish. The resulting ELQ-containing extruded polymer thin-film was allowed to cool and peeled off the Petri dish lid using a laboratory spatula.
Preparation of extruded ELQ HDPE films
HDPE pellets (McMaster-Carr, 7202N11) were cryogenically milled into a flowable powder by freezing the pellets with liquid nitrogen and then milling the cold HDPE through a Retsch Mill with a 1.0 mm sieve to maximize blending efficiency and uniformity and to increase the rate of melting of the HDPE. The dried HDPE powder and ELQ powders were weighed, combined and physically mixed until uniform. HDPE powder and ELQ mixtures were placed into a feeder and fed into a Haake MiniLab (Thermo Electron) twin screw extruder with a barrel temperature of 150 °C. The blend was extruded through a 3-mm-diameter nozzle, collected and allowed to cool in ambient conditions. The collected filaments for each formulation were stored in a sealed bag until use. Each of the collected formulation filaments was cut to sizes of roughly 5–7.5 cm in length. The platens on a Model C, Carver press with two stainless steel sheets covered in aluminium foil and a stainless steel spacer (with a square cavity of 10.16 cm × 10.16 cm × 0.08 cm thick) were preheated to 150 °C. The plates were then removed from the press and 7.5 g of the 5–7.5 cm cut filament were placed in the middle of the plates (with one plate on the bottom and insert around the filaments and the second plate on top of the filaments). The mould was placed back into the Carver press and gently compressed for 1.5 min. The sample was then pressed to 2267.962 kg (5,000 lbs) for 1 min and 4535.924 kg (10,000 lbs) for 30 s. The plates were removed from the Carver press and allowed to cool in ambient conditions for 15 min with a weight on top of the mould. The films were deburred and stored in Ziploc bags until tarsal-contact assays were performed.
Ethics statement
Human whole blood was ethically sourced from healthy volunteers, and their research use was in accord with the terms of the informed consent under a protocol approved by the Institutional Review Board (IRB 120160613, Research Blood Components).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.