Mice
Mice were bred and maintained in groups of five animals per cage at the CNIC under specific pathogen-free conditions. We used proatherogenic background B6.129P2-Apoetm1Unc/J mice (The Jackson Laboratory, strain 002052, Apoe−/−); B6.129S7-Ldlrtm1Her/J mice (The Jackson Laboratory, strain 002207, Ldlr−/−) backcrossed with B6.SJL-Ptprca Pepcb/BoyJ mice expressing the CD45.1 congenic marker (The Jackson Laboratory, strain 002014, B6 CD45.1); B6.129S7-Rag1tm1Mom/J mice (The Jackson Laboratory, strain 002216, Rag1–/–); B6.Cg-Rptortm1.1Dmsa/J mice (The Jackson Laboratory, strain 013188, RaploxP) were mated with B6.129P2-Lyz2tm1(cre)Ifo/J mice (The Jackson Laboratory, strain 004781, Lyz2-cre) to generate Lyz2ΔRaptor (Cre+) and control Raptorfl/fl (Cre−) littermates used as controls. Sperm from B6.Nischtm1a(EUCOMM)Hmgu>/H mice (Mary Lyon Centre at MRC Harwell, UK; EM:08808) was used for in vitro fertilization of ROSA-Flpe mice (Gt(ROSA)26Sortm1(FLP1)Dym) to generate Nischfl/fl mice (tm1c). Nischfl/fl mice were mated with Lyz2-cre (B6.129P2-Lyz2tm1(cre)Ifo/J) mice to generate Lyz2ΔNisch (Cre+) and control Nischfl/fl (Cre−) littermates used as controls. Mice were 7- to 12-week-old at the initiation of experiments. Littermates of the same sex were randomly assigned to experimental groups. Investigators were blinded to group allocation during data collection and analysis to reduce bias. Animal studies were approved by the local ethics committee at CNIC, UAM and Comunidad de Madrid, PROEX 300.4/21 and PROEX 119.6/22. All animal procedures conformed to European Union Directive 2010/63EU and Recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes, enforced by Spanish law under Real Decreto 53/2013. Mice were fed different diets, including chow, HC (10% fat, 0.75% cholesterol, SSNIFF S9167-E011) and HC/HC (HC + 1% choline, SSNIFF S9167-E016), as indicated in each experiment. ImP (400 μg per mouse per day, Biogen Cientifica BA-F-3185.0001) was administered in the drinking water. AGN192403 (30 μg per mouse per day, Labclinics B6583-10) was administered in the drinking water.
Antibiotic administration
For ablation of intestinal bacteria, an antibiotic cocktail of 1 g l−1 each of ampicillin (Normon), neomycin sulfate (Sigma Aldrich) and metronidazole (Sanofi), 0.5 g l−1 vancomycin (Normon) and 4 mg ml−1 sucralose (Sigma Aldrich) dissolved in water was used and replenished every three days as described52.
Quantification of lesions in the aorta
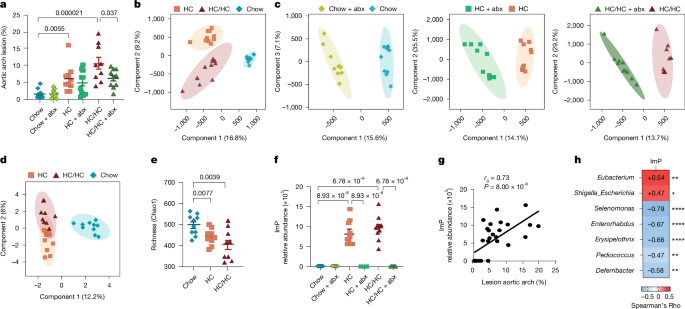
For atherosclerotic lesion measurements, Apoe−/− or Ldlr−/− male or female mice were fed a chow or high-cholesterol diet for 4, 8 or 12 weeks, as indicated in each experiment. Blood was collected from submandibularly punctured mice. Next, mice were perfused with 10 ml phosphate buffered saline (PBS) via cardiac puncture to remove blood contamination from vascular tissue. The aortas were dissected and fixed in formaldehyde 4% w/w (PanReac, AppliChem) at room temperature overnight. Then, the exposed aortas were stained for lipid depositions with Oil Red O (Sigma Aldrich) prior en face assay assessment. In brief, after fixation, aortas were washed with 60% isopropanol and stained for one hour with 2.5 mg ml−1 of Oil Red O. After two washing steps of 5 min with isopropanol 60%, aortas were stored in PBS-azide 0.05%. Images were taken with Leica Nikon camera magnifier and atherosclerotic lesions in the aorta were quantified by calculating the ratio of the lesion area to the total surface area. This measurement was carried out using an automated method that quantifies lipid accumulation in the lesions by applying colour thresholding to the Oil red O-positive areas. The threshold was set by the researcher and remained consistent within the same staining batch, as described53. Analysis of the images was performed using ImageJ software.
Aortic roots histology
Perfused hearts were fixed with 4% formaldehyde for 48 h at room temperature. For paraffin sectioning, fixed hearts were incubated overnight in 70% ethanol and embedded in paraffin blocks. Four-micrometre sections of the aortic roots were cut and dried overnight at 37 °C. The trichrome protocol used was a standard Masson’s trichrome protocol using Weigert’s haematoxylin for nuclei and light green as a collagen stain. Trichrome stain was used to analyse plaque and necrotic core (identified as unstrained regions inside plaque). T cell staining in the heart aortic roots was performed by immunohistochemistry using a CD3 antibody (Invitrogen, MA1-90582). Macrophage staining in the atheroma plaque was performed by immunohistochemestry using a MAC2 antibody (Invitrogen, 14-5302-85). Caspase-3 staining was carried out in the heart aortic roots using anti-caspase-3 antibody (Cell Signaling Technology, 9661). Staining was performed in an automated autostainer (Autostainer Plus, Dako). As secondary antibodies we used anti-rabbit (Dako Envision K4003) for CD3 and caspase-3 staining and rabbit anti-rat (Abcam Ab6734) for MAC2. The digitalized images were obtained using the Carl Zeiss Axio Scan Z1 scanner. Analysis of the images was performed using NDPview 2 and ImageJ software.
Caecal content collection
At the culling, the caecum was resected and rinsed with PBS to collect its contents, which were stored at −80 °C until sample processing.
Serum analysis
Blood samples were obtained by cardiac puncture. The serum was collected after blood centrifugation at 1,000g for 10 min and frozen at −80 °C. Serum biochemical parameters were analysed with a Dimension RxL Max automated analyser the day after the extraction. Glucose and protein content were measured with Dimension RxL Max automated analyser.
LC–MS untargeted metabolomics
Materials
LC–MS grade acetonitrile was from Fluka Analytical; methanol and water were from Fischer Scientific; ethanol was from Merck and formic acid was from Honeywell Fluka.
Sample preparation
Plasma samples (50 µl) were prepared as described54. In brief, proteins were removed by adding a cold (−20 °C) mixture of methanol:ethanol (1:1, v/v) in a ratio 5:1 (solvent:sample) and by storing the samples on ice for 20 min. Blank sample was prepared following the same extraction procedure, adding only the solvents to a 1.5 ml Eppendorf tube. Quality control samples (QCs) were prepared by pooling 100 µl plasma samples from independent Apoe−/− mice and following the same extraction procedures. Samples, QCs and blank, were centrifuged at 16,400g for 20 min 4 °C and the supernatant was transferred in a 1.5 ml Eppendorf tube. Supernatants were subsequently dried-out in speedvac (Savant SPD131DDA concentrator, Savant RVT5105 refrigerated vapour trap and OFP400 vacuum pump, Thermo Fisher Scientific) for 2 h at room temperature. Before LC–MS analysis, the dried samples and QCs were resuspended in 50 µl and 100 µl of a mixture composed of acetonitrile:H2O (90:10, v:v) by constant shaking at 1,000 rpm for 10 min at 8 °C and then centrifuged at 16,400g for 5 min to remove insoluble material. The soluble part was placed in the insert of the LC–MS vial.
LC–MS analysis
Metabolomics untargeted analysis was performed using an Ultimate 3000 HPLC system consisting of a degasser, two binary pumps, and autosampler with thermostat, maintained at 8 °C (Thermo Fisher Scientific) coupled to a LTQ Orbitrap XL Hybrid Ion Trap-Orbitrap Mass Spectrometer (Thermo Fisher Scientific). The samples (5 µl) were injected onto a Merck SeQuant ZIC-HILIC column (150 × 1 mm, 3.5 µm), which was maintained thermostatically at 45 °C, and metabolites were eluted at 180 µl min−1 with solvent A composed of water with 0.1% formic acid, and solvent B composed of acetonitrile with 0.1% formic acid. The gradient started from 90% to 25% of B in 15 min, keeping constant for 3 min and returned to starting conditions in 0.1 min, finally by keeping the re-equilibration at 90% of B for 11.9 min. Data were collected in positive ESI ion mode. The mass spectrometer operated in full scan mode from 70 to 1,000 m/z at 60,000 resolution. MS/MS spectra were collected in data-dependent mode via collision induced dissociation (CID) in the ion trap. Samples were analysed in a randomized order. QCs were analysed at the beginning, at the end and every six samples.
Data handling
Generated data were firstly aligned using Compound Discoverer (Thermo Fisher Scientific); signals were extracted and grouped into features (isotopic traces from a single analyte at a particular charge state) using the Metaboprofiler node in Compound discoverer (open source plug-in freely available from OpenMS; https://openms.de/) as described55. Features were then filtered by presence keeping the entities that were present in at least 80% of the samples from the same group. Missing values were imputed using the k-nearest neigbours method or by limit of detection (LoD) (1/5 of the minimum positive value of each variable) according to the percentage of missing values of the feature within the group in MetaboAnalyst v.5 (https://www.metaboanalyst.ca/MetaboAnalyst/home.xhtml)56. Data were autoscaled and log-transformed. Metabolites were annotated by searching their accurate experimental mass against the Ceu Mass Mediator (http://ceumass.eps.uspceu.es/)57 and the resulting data matrix handled by Turboputative (https://proteomics.cnic.es/TurboPutative/)58. Identification of ImP was then confirmed by using the reference standard (Sigma Aldrich 77951) in a subsequent LC–MS/MS analysis following higher-energy collisional dissociation by using 50 eV as collisional energy.
Flow cytometry analysis
For blood samples, single-cell suspensions were stained for 30 min at 4 °C with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies). After washing with PBS, cells were stained in FACS buffer containing anti-CD16/32 (BioXcell), 3% FBS and 0.05% EDTA with the corresponding antibody cocktail for 30 min on ice. Cells were stained with a cocktail of antibodies against CD45-APCCy7 (Biolegend), CD11b-BV605 (Biolegend), CD11c-BV650 (Biolegend), Ly6C-BV711 (Biolegend), LY6G-APC (BD Biosciences), CD8-PE (eBioscience), CD90-BV785 (BD Biosciences), MHCII-AF700 (Biolegend), B220-PerCP5.5 (Biolegend), SiglecH-FITC (eBioscience), CD4-PeCy7 (Biolegend). In another set of experiments, cells were stained with, CD45-APCCy7 (Biolegend), LY6G-V450 (BD Biosciences), CD11b-ef660 (eBioscience), CD11c-PE (Biolegend), B220-PerCP (Biolegend), CD90-UV805 (Fisher scientific), XCR1-BV785 (Biolegend), CD206-eF450 (Biolegend), MHCII-UV737 (BD Biosciences), F4/80-PerCp5.5 (Biolegend), Rorγt-APC (Biolegend), T-bet-PECF594 (BD Biosciences), Ly6C-BV711 (Biolegend), CD86-PECy7 (Biolegend), FOXP3-FITC (Biolegend), CD4-BV570 (Biolegend). Cells were washed again and resuspended in FACS buffer for data acquisition using an LSRFortessa SORP (Becton Dickinson) or a FACSymphony (Becton Dickinson) flow cytometry equipment and analysed with FlowJo software version 10 (TreeStar). The gating strategies used for these analyses are detailed in Supplementary Fig. 1.
To perform flow cytometry analysis of cells infiltrating the aorta, mice were perfused with 10 ml PBS via cardiac puncture to remove blood contamination from vascular tissue. Aortas were kept in cold Dulbecco’s modified Eagle medium (DMEM) to be digested. Perivascular fat was removed and the thoracic aorta with arch was opened longitudinally and cut into smaller pieces that were incubated for 30 min at 37 °C in a water bath in digestion buffer (Collagenase A (25 mg ml−1), (Roche/Sigma 10103586001), Dispase II (25 mg ml−1), (Roche/Sigma 04942078001), DNase I (250 μg ml−1) (Roche/Sigma 10104159001) elastase (25 μg ml−1) and Libersase TL (0.2 Wünsch units per ml, 5401119001)). After incubation time the digested tissue was mixed by pipetting, filtered through a 70-μm strainer and spinned at 400g 5 min at 4 °C. Single cells were stained with a cocktail of antibodies against CD45-APCCy7 (Biolegend), CD11b-BV605 (Biolegend), CD11c-BV650 (Biolegend), Ly6C-BV711 (Biolegend), LY6G-APC (BD Biosciences), CD8-PE (eBioscience), CD90-BV785 (BD Biosciences), MHCII-AF700 (Biolegend), B220-PerCP5.5 (Biolegend), SiglecH-FITC (eBioscience), CD4-PeCy7 (Biolegend). Depending on the experimental setting, cells were stained with CD45-APCCy7 (Biolegend), LY6G-V450 (BD Biosciences), CD11b-ef660 (eBioscience), CD45-APCCy7 (Biolegend), LY6G-V450 (BD Biosciences), CD11b-ef660 (eBioscience), CD11c-PE (Biolegend), B220-PerCP (Biolegend), CD90-UV805 (Fisher scientific), XCR1-BV785 (Biolegend), CD206-eF450 (Biolegend), MHCII-UV737 (BD Biosciences), F4/80-PerCp5.5 (Biolegend), Rorγt-APC (Biolegend), T-bet-PECF594 (BD Biosciences), Ly6C-BV711 (Biolegend), CD86-PECy7 (Biolegend), FOXP3-FITC (Biolegend), CD4-BV570 (Biolegend), CD31-PE (Biolegend) and VCAM-AF594 (Biotechne). Cells were washed and resuspended in FACS buffer for data acquisition using a LSRFortessa SORP (Becton Dickinson) or a FACSymphony (Becton Dickinson) flow cytometry equipment and analysed with FlowJo software version 10 (TreeStar). Gating strategies for B cells, T cells, TH1 and Treg cells are shown in Supplementary Fig. 2, and those for macrophage subsets are shown in Supplementary Fig. 3.
Study populations
PESA cohort
This study was conducted in a subset of participants (n = 400) from the PESA study59. The PESA-CNIC-Santander (NCT01410318) is an ongoing observational prospective cohort study of 4,184 asymptomatic employees of the Santander Bank in Madrid (from 40 to 54 years of age and free of known CVD at baseline in 2009). In addition to the exclusion criteria followed in the main study2, participants taking antibiotics in the three months prior to the sample collection, with known type 2 diabetes or those treated for diabetes and/or intestinal disorders were excluded. This enabled us to remove possible confounding effects due to the role of ImP in diabetes and insulin resistance15 and the modification of gut microbiota possibly affecting the production of ImP. Subclinical atherosclerosis was assessed by imaging studies including 2D and 3D vascular ultrasonography of carotid and iliofemoral arteries and presence of coronary artery calcium assessed by CT scan, as previously described59. Participants with subclinical atherosclerosis underwent a whole body 18F-FDG PET/MRI study to characterize arterial 18F-FDG uptake and bone marrow metabolic activity, as described20,21. The cardiovascular risk score used included the European Society of Cardiology SCORE (Systematic Coronary Risk Evaluation), which calculates 10-year risk of fatal CVD1. Fasting blood test included biochemistry and determination of hs-CRP. In this study, hypertension was defined as systolic >130 mmHg and/or diastolic >85 mmHg and/or individuals taking antihypertensive drugs. Dyslipidaemia was defined as total cholesterol ≥240 mg dl−1, LDL-cholesterol ≥160 mg dl−1, HDL-cholesterol <40 mg dl−1, or use of lipid-lowering drugs. Anthropometry and bioelectrical impedance analysis assessments were performed during the same appointment for each participant. Height and weight were measured using calibrated equipment (Tanita BC-545N,) with participants wearing underwear and barefoot. BMI was calculated as weight (in kg) divided by height (in metres) squared. Extent of atherosclerosis was defined by a summed ordinal variable that is calculated by the number of affected vascular sites into a sum of total points as follows: (1) carotid atherosclerosis (points: 0, no plaque; 1, 1 plaque; 2, 2 plaques; 3, 3 or more plaques) as estimated by 3D vascular ultrasonography and summed for both left and right carotid artery; (2) femoral atherosclerosis (points: 0, no plaque; 1, 1 plaque; 2, 2 plaques; 3, 3 or more plaques) as estimated by 3D vascular ultrasonography and summed for both left and right femoral artery; (3) presence of calcification in the ascending or descending aorta (points: 0, no; 1, yes); (4) CAC score (points: 0, 0; 1, 0.1–99; 2, 100–399; 3, 400–maximum). For the metabolomic analysis, peripheral blood samples collected after overnight fasting were centrifuged at 2,750g at room temperature for 10 min to obtain plasma that was further aliquoted and stored at −80 °C. For 16S rDNA amplicon gene sequencing analysis, faeces were collected in collection cups and immediately frozen at −80 °C until further analysis. The institutional ethics committee approved the study protocol, and all participants were provided with written informed consent. Characteristics of the participants are listed in Supplementary Tables 1a,b and 3.
IGT cohort
To validate the findings from the PESA study, a subset of the IGT cohort was used (Ethics 560-13). Subject and method design from the validation cohort have been described elsewhere16. In brief, the study population comprises men and women aged 50–64 years from the Gothenburg area (Sweden), recruited on a random basis from the Swedish population register and included based on their glucose status. The applied exclusion criteria included: known diabetes, inflammatory diseases (for example, Crohn′s disease, ulcerative colitis or rheumatic diseases), treatment with steroids or immunomodulatory drugs, cancer (unless relapse free for the preceding five years), cognitive dysfunction, treatment for infectious diseases and with antibiotics in the past three months, inability to understand written and spoken Swedish as well as individuals born outside Sweden. The participants were invited by letter and gave all informed consent. Atherosclerosis was assessed by ultrasound imaging of carotid arteries, using a standardized protocol including a Siemens Acuson S2000 ultrasound scanner, and by presence of coronary artery calcium assessed by CT scan, as previously described16. Systolic blood pressure was measured twice with an automatic device (Omron M10‐IT, Omron Health Care Company) and the mean of the measurements was used. A questionnaire was used to collect detailed information on medication and family history. A venous blood sample (100 ml) was collected from participants after an overnight fast and was used for immediate biochemical analysis of glucose, glycated haemoglobin and total cholesterol. SCORE2 (systematic coronary risk evaluation) was calculated as described1. Body weight, height, waist and hip circumference were measured on calibrated equipment with participants dressed in light clothing without shoes and according to current WHO recommendations. Extent of atherosclerosis was defined by a summed ordinal variable that is calculated by the number of affected vascular sites into a sum of total points as follows: (1) carotid atherosclerosis (points: 0, no plaque; 1, at least 1 plaque at either left or right carotid; 2, plaques at both vessels); (2) CAC score (points: 0, 0; 1, 0.1–99; 2, 100–399; 3, 400–maximum). Characteristics of the participants are listed in Supplementary Table 2a,b.
Targeted metabolomics
Plasma levels of ImP, urocanic acid and histidine were quantified using ultra high-performance liquid chromatography (UHPLC) coupled to tandem mass spectrometry (LC–MS/MS). Analyses were performed in two different laboratories in Spain (PESA cohort) and in Sweden (IGT cohort). Both laboratories have used fully validated LC–MS/MS methods.
The determination of ImP in plasma samples from Apoe−/− mice fed a chow or high-cholesterol diet with and without ImP (400 μg per mouse per day) and/or AGN192403 (30 μg per mouse per day) in the drinking water was carried out by following the method described below for the human study, specifically for the PESA cohort.
Working solutions and standards
For the PESA cohort, individual 1,000 ppm stock solutions of ImP (Sigma Aldrich 77951), urocanic acid (urocanic acid-1,2,3-13C3, Sigma Aldrich, 709638) and histidine (l-histidine-d3 hydrochloride monohydrate, Sigma Aldrich, 791318) were prepared in ultrapure water and stored at −20 °C. Their respective dilutions were then prepared in methanol:ethanol (1:1, v/v) and stored at 4 °C. For the IGT cohort, histidine (histidine-d5 15N3, Cambridge Isotope Lab), ImP and urocanic acid (ImP-13C3 and urocanate-13C3, Astra Zeneca) were prepared in acetonitrile.
Metabolite quantification
For the PESA cohort, 65 µl of methanol:ethanol (1:1, v/v) were added to 50 µl of plasma sample and 35 µl of isotopically labelled internal standards in methanol:ethanol (urocanic acid and histidine). Samples were vortex-mixed for 10 s and then centrifuged at 13,000 rpm for 20 min at 4 °C. One-hundred microlitres of supernatant were taken and transferred to liquid chromatography vials for the analysis. Quantification of the metabolites was performed by using an UHPLC system 1290 Infinity series coupled to a triple quadrupole (QqQ) 6460 MS from Agilent Technologies. In brief, 1 µl of the sample was injected onto an InfinityLab Poroshell 120 HILIC-Z column (2.7 μm, 150 × 2.1 mm, Agilent Technologies) with a flow rate of 0.50 ml min−1. Mobile phase A contained 20 mM ammonium formate in ultrapure water at pH 3, whereas mobile phase B contained 20 mM aqueous ammonium formate at pH 3 in acetonitrile:H2O (9:1, v/v). The initial conditions at time 0 were 100% B, decreasing to 70% at 11.5 min. The gradient was then increased to 100% B at time 12.0 min and held until the total run time of 15 min. Mass spectrometry data were acquired in positive ionization mode. Raw signals for multiple reaction monitoring transitions were checked and peaks corresponding to all the targeted compounds were integrated by MassHunter Quantitative B.10.00 (Agilent Technologies). Multiple reaction monitoring transitions (collision energy, eV) were as follows: 141>123 (10) and 141>81 (26) for ImP; 139>121 (10) and 139>93 (26) for urocanic acid; 142>124 (10) and 142>41 (26) for labelled urocanic acid; 156>110 (10) and 156>83 (30), for histidine; and 159>113 (10) and 159>86 (30) for labelled histidine. Quantification of ImP concentration was performed by generating a standard curve with known concentrations of this metabolite. Histidine and urocanic acid were instead quantified by using internal standards added to plasma samples as stated above. Metabolite standards were analysed alongside plasma samples using the LC–MS/MS method. Dilution of the plasma during sample preparation was also taken into account for quantification.
For the IGT cohort, determination of ImP, urocanate and histidine levels in plasma were performed as described previously with minor modifications15. In brief, 25 µl of plasma samples were extracted using 6 volumes of acetonitrile containing 100 nM of internal standards before drying the samples under a flow of nitrogen. Then, the samples were reconstituted with 5% HCl (37%) in 1-butanol, subjected to n-butyl ester derivatization and finally reconstituted in 150 µl water:acetonitrile (9:1). ImP, urocanate and histidine levels were determined by UHPLC–MS/MS analysis using multiple reaction monitoring of the transitions 197.2>81.2, 195>93 and 213.2>110.1, respectively. For the internal standards, the transitions 200.2>82.0, 198.2>95.0 and 220.3>118.1 were used. The analytical system consisted of an Acquity UPLC I-class system coupled to a Xevo TQ-XS triple quadrupole mass spectrometer (Waters). Sample volume (2 µl) was injected onto a C18 BEH column (2.1 × 50 mm with 1.7-µm particles, Waters) and separated using a gradient consisting of water with 0.1% formic acid (A-phase) and acetonitrile with 0.1% formic acid (B-phase). Quantifications were performed using a standard curve with known concentrations of ImP, urocanate and histidine.
DNA extraction, 16S rDNA amplicon sequencing and microbiota data analysis
For mice caecum samples, total DNA was extracted using the MasterPure Complete DNA & RNA Purification Kit (Epicentre) with some modifications, as previously described60. DNA concentrations were normalized using a Qubit 2.0 Fluorometer (Life Technology) and specific amplicon of the 16S rDNA gene (V3–V4 region) was generated following the 16S Metagenomic Sequencing Library Preparation Illumina protocol. The multiplexing step was performed using Nextera XT Index Kit (Illumina) and PCR amplicon-product was checked on a bioanalyzer DNA 1000 chip (Agilent Technologies). The samples were pooled in equimolar amounts and sequenced with the MiSeq Reagent kit v.3, on a MiSeq Illumina platform (FISABIO sequencing service).
For human samples, faecal DNA was extracted using a commercially available kit (E.Z.N.A stool DNA kit, Omega Biotek)61. Next, amplicon library of the V4 region within the 16S rDNA gene were generated and both ends of the fragments were sequenced using an Illumina MiSeq by the Microbiome Core of the University of Michigan62.
In both studies, to rule out and control for potential reagent contamination, the reagents for DNA extraction and PCR amplification were also sequenced as controls. For data analysis of microbiota from mice and humans, the paired-end sequences were curated, binned into operational taxonomic units at >97% identity level, and annotated with SILVA release v.132 using DADA263 for mice data and v.138 and RDP version 18 databases using Mothur (v.1.40.5)62,64 as previously described61 for human data. Spearman rank correlation coefficients between ImP level and bacterial abundance were determined using otu.association function of Mothur62,64.
Dietary pattern assessment
Dietary intake data were collected via semi-quantitative 136-item food-frequency questionnaire validated for the Spanish population65. Foods reported during the dietary assessment were classified into 44 food groups based on the similarity of nutrients profile, averaged over the days to derive habitual intake and normalized by the BMI of each participant. Next, a PCA was performed in SPSS V.23.0 (SPSS) to identify common underlying dimensions (factors or patterns) of food consumption by deriving factor loadings for each predefined food group. Factors were subsequently rotated using a Varimax procedure to maintain uncorrelated factors. Analysis of eigenvalues, scree plot, and the interpretability of the factor solution were used to support a final decision on retaining a 5-factor solution, where each factor had an eigenvalue >0.3. According to the observed factor loadings of the food groups, we named the first factor Mediterranean dietary pattern (enriched in vegetables, whole grains, and fish), the second and third factors as Breakfast dietary patterns (Breakfast1: enriched in cereals, tea, low fat dairy, and Breakfast2: mainly composed of bread, egg and coffee), and the fourth factor the Western dietary pattern (enriched in red meat, sugar, snacks and refined grains) and the fifth factor the Social dietary pattern (enriched in soft drinks and spirits).
scRNA-seq
Apoe−/− 8-week-old male mice were treated in the drinking water with ImP (400 μg per mouse per day) for 4 or 8 weeks. Aortas were collected and digested as described above. To obtain enough cell numbers, three aortas per condition were pooled. Each sample was labelled with a cell multiplexing oligo (CMO) using the CMO labelling for scRNA-seq Protocol from 10X Genomics. Cells labelled with CMOs were washed, labelled with SytoxGreen and Hoechst to ensure cell viability, and live cells were sorted with a FACs Aria cell sorter. Samples were pooled and scRNA-seq was performed following the Chromium Next GEM Single Cell 3′ v.3.1 with feature barcode technology for cell multiplexing protocol (10x Genomics). In brief, cells were first counted and checked their viability using the Countess 3 cell counter (Thermofisher). Next, cells were loaded onto a 10X Genomics chip of the Chromium Controller (10X Genomics). After cDNA amplification, gene expression and CMO libraries were generated and sequenced using an Illumina NextSeq 2000 sequencer.
Raw sequencing data processing was performed from FASTQ file from each port using Cell Ranger (v.6.1.2) with default parameters and the mm10 (GRCm38.p6) mouse genome reference provided by 10X Genomics. Obtained raw unique molecular identifier (UMI) count matrices of valid barcoded cells for each port were loaded into R (v.4.1.2) for further analyses using Bioconductor packages66 and Seurat (v.4.0.6)67. First, cells were filtered out according to the total number of UMI counts (≤1,000 and ≥50,000), total number of detected genes (≤200 and ≥6,000), and percentage of mitochondrial UMI counts (≥7.5%). Then, resulted cells were demultiplexed using the cellhashR R package (v.1.0.2)68 using htodemux, bff_cluster, gmm_demux, multiseq, and dropletutils methods. Consensus classification was used for further analyses. Once the final set of cells was obtained, transcriptional expression values were normalized to total UMI counts per cell multiplied by a scaling factor of 10,000 and natural log-transformed (NormalizeData function). Highly variable genes were defined using the VST method as implemented in Seurat setting. A total of 2,000 highly variable genes and PCA modelling was used to reduce this dimensional space after scaling log-transformed expression values. In this step, variability associated with total number of counts was regressed out using the ScaleData function with linear models. Elbow plot method was used to determine the number of principal components for downstream analyses (25 principal components). Then, cells were clustered using the Louvain algorithm and visualized using the uniform manifold approximation and projection for dimension reduction (UMAP) algorithm with the first 25 principal components as input. Main clusters were identified by using known markers and computing differentially expressed genes with Wilcoxon rank sum test using the presto R package (v.1.0.0) (Extended Data Fig. 4). Cell proportions were calculated for each condition in every cell population, and a two-proportions Z-test using the prop.test R function was used to determine significance.
Differential expression analyses between conditions were independently performed on every cell population comparing ImP vs control for each timepoint (4 or 8 weeks) using the MAST R package (v.1.20.0). Genes were ranked according to log fold changes, and resulting ranks were used as input for the fgsea R package along with the Hallmark gene sets from the Molecular Signature Data Base (MSigDB; https://www.gsea-msigdb.org/gsea/msigdb). In order to identify specific cell subpopulations within every general cell type, each population was separated and independently analysed using the same workflow explained above. Subclusters were identified by using known markers and calculating differentially expressed genes with the presto R package. Finally, an overrepresentation analysis considering gene markers for every identified subcluster was performed along with the GO database (https://www.geneontology.org/) in order to interpret the transcriptional state of each subpopulation.
Cell lines
MAECs were isolated from mouse thoracic aortas as described69. Cells were cultured in Medium 199 (Gibco, Invitrogen Life Technologies) + 20% fetal bovine serum + penicillin/streptomycin 2 mM + glutamine 2 mM + HEPES 10 mM + endothelial cell growth supplement 30 μg ml−1 + Heparin 100 mg ml−1, all from Sigma Aldrich, under 5% CO2 at 37 °C. MEF cell lines were isolated from 13.5 days post coitum from embryos using standard protocol. Each embryo was dissected into 10 ml of sterile PBS, voided of its internal organs, head, and legs. After 30 min incubation with gentle shaking at 37 °C with 5 ml 0.1% trypsin, cells were plated in two 100 mm dishes and incubated for 24–48 h. To establish the immortalized MEF lines, early passage MEFs were seeded in 60 mm plates and infected with 105 IU ml−1 packaged retrovirus carrying the human papillomavirus (HPV) 16 E6/E7 genes. Selection was performed with 400 μg ml−1 of G418 during 10 days.
L929 cell line (ATCC CCL-1TM) used for production of the M-CSF supernatant, was grown on 175 cm2 cell culture flasks (Stemcell) and resuspended in RPMI 1640 (Sigma) supplemented with 10% heat-inactivated fetal calf serum (FCS, Sigma), 1 mM pyruvate (Lonza), 100 µM non-essential aminoacids (Thermo Fisher Scientific), 2 mM l-glutamine, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin (all three from Lonza), herein called R10. Supernatants were obtained by filtering 15-days long cultures through 0.22 μm Stericup Filter units (Merck Millipore) and were used to subsequently supplement the medium for the generation of BMDMs.
Mouse BMDMs were generated as described70, with some modifications. Bone marrow cells from WT C57BL/6J mice were cultured in RPMI 1640 supplemented with 30% M-CSF obtained from L929 cell line and 10% FBS, 100 μg ml−1 penicillin, 100 μg ml−1 streptomycin, 10 mM HEPES, 1 nM sodium pyruvate (all from Gibco) during 5 days in sterile, but not tissue culture-treated, 10-cm Petri dishes.
Bulk RNA-seq
Cultured BMDMs, MEFs and MAECs were plated in equal number and rested overnight. Specifically, for BMDMs and MEFs, 6 × 105 cells per well were plated in 6-well plates (2,000 µl final volume, Corning), and for MAECs 1 × 105 cells per well were plated in p24 (1,000 µl final volume, Corning). Cells were treated in vitro with or without ImP (10 μg ml−1) for 1 or 2 h. Total RNA was isolated from BMDMs, MEFs (QIAamp DNA Mini Kit) and MAECs (miRNeasy Micro Kit). Bulk RNA-seq experiments were performed in the Genomics Unit of the CNIC. For the BMDM and MEF samples, RNA-seq libraries were prepared using the NEBNext Ultra II Directional RNA Library preparation kit (New England Biolabs) according to the manufacturer’s instructions. For MAECs samples, libraries were prepared using the NEBNext Single Cell/Low Input RNA Library Prep Kit for Illumina (New England Biolabs) according to the manufacturer’s instructions. All libraries were sequenced in a Nextseq 2000 (Illumina). FastQ files for each sample were obtained using bcl2fastq v.2 2.20 software (Illumina). The number of reads per sample was between 11 and 21 million. Reads were preprocessed according to the type of library preparation using cutadapt to trim adapters and FastQC to assess read quality. In the case of directional RNA-seq reads (BMDMs and MEFs), Illumina adaptor sequences were removed, and only reads longer than 30 bp were kept as valid. For MAECs, in addition to Illumina adaptor sequences, adaptors from the low input NEBNext protocol were detected and removed and only reads longer than 30 bp were kept as valid. Resulting reads were mapped to the reference transcriptome GRCm38.102 using STAR71 and gene expression levels were estimated using RSEM72. Further analyses were performed in R (v.4.3.2). In brief, counts per million were calculated, and size factors for inter-sample normalization were determined using the trimmed mean of M values method. Then, log-normalized data considering only genes with ≥20 counts in 3 samples were used for differential expression analysis, making all possible comparisons within the same cell type using the DESeq2 R package73. Gene-set enrichment analysis was performed using the fgsea R package comparing 1 h and 2 h after ImP stimulation with 0 h (unstimulated) within each cell type. Genes were ranked in each comparison according to logFC, and fgsea was used along with the Hallmark gene sets from the Molecular Signature Data Base (MSigDB; https://www.gsea-msigdb.org/gsea/msigdb). Finally, normalized enrichment scores of significant selected pathways were represented as a heat map using the ComplexHeatmap R package74.
Proteomics and phosphoproteomics by isobaric labelling
Cultured BMDMs and MEFs were plated at 1 × 106 cells per well in 6-well plates (Corning), 2 ml final volume, and rested overnight in RPMI 1% medium. Cells were treated in vitro with or without ImP (10 μM) and co-incubated or not with AGN192403 (1 μM, I1R antagonist) for 180 min. Cell culture monolayers were washed with PBS, and protein extracts were obtained with lysis buffer (50 mM Tris-HCl pH 7.6, 2% SDS, 10 mM Tris-(2-carboxyethyl)phosphine (TCEP)) containing protease and phosphatase inhibitor cocktails (Complete Mini EDTA-free and phosSTOP, Roche). Samples were boiled for 5 min and incubated for 20 min at room temperature for solubilization and reduction of the proteins, and after centrifugation at 15,000g for 15 min, supernatants were recovered, and protein concentration was determined by using a RC DC Protein Assay Kit (BioRad Laboratories).
Proteome and phosphoproteome analyses were carried out using 18-plex isobaric TMT labelling as described75. Statistical analysis of quantitative data was performed using iSanXoT76, and the comparative analysis between conditions was performed using the normalized values \({X}_{p}^{m}\) and \({X}_{q}^{m}\), which represent the binary logarithm of the ratio between the abundance in sample m and its control for peptide p and protein q, respectively. GSEA77 using Xp values of peptides was used to evaluate the phosphorylation levels in proteins of the mTOR signalling pathway among conditions (that is, cells stimulated with ImP versus unstimulated). In addition, a Wilcoxon signed rank test was performed using paired phosphopeptide abundance Xp values to determine the effects of AGN192403 in the phosphorylation levels of the mTOR pathway among cells treated with ImP and co-incubated or not with AGN192403.
p-S6 staining by flow cytometry
Cultured MEFs and BMDMs were plated in equal numbers (6 × 105 cells per well) in 6-well plates (2,000 µl final volume, Corning) and rested overnight. Cells were treated in vitro with or without ImP (10 μg ml−1) co-incubated with Rapamycin (10 nM), AGN192403 (1 μM, I1R antagonist), Idazoxan (0.29 nM, imidazoline receptor and α2-adrenergic antagonist) or Yohimbine (0.28 nM, α2 antagonist). After 24 h, cells were collected and labelled with p-S6-PE antibody (Cell Signalling 5316) according to the manufacturer protocol and the activation was measured by flow cytometry.
Peritoneal macrophages collection
Peritoneum cells were isolated by lavage with 5 ml of cold PBS. Cells were plated and non-adherent cells were washed with PBS 1 h later. Adherent cells were defined as peritoneal macrophages, which were routinely controlled by flow cytometry. p-S6 staining by flow cytometry was assessed as described above.
TNF measurement
Cultured MEFs and BMDMs were plated in equal numbers (1 × 105 cells per well) in 96-well plates (200 µl final volume, Corning) and rested overnight. Cells were treated in vitro with and without ImP (10 μM) and co-incubated with Rapamycin (10 nM), AGN192403 (1 μM, I1R antagonist), Idazoxan (0.29 nM, imidazoline receptor and α2 antagonist) or Yohimbine (2.8 nM, α2 antagonist). After 24 h, supernatant was collected, and TNF concentration was quantified with mouse TNF DuoSet ELISA (DY410) according to the manufacturer’s instructions.
MCP1 measurement
Cultured MEFs were plated (1 × 105 cells per well) in 96-well plates (200 µl final volume, Corning) and rested overnight. Cells were treated in vitro with and without ImP (10 μM). After 24 h supernatant was collected, and MCP1 concentration was quantified with mouse CCL2/JE/MCP1 DuoSet ELISA (DY479) according to the manufacturer’s instructions.
Transwell
Migration of circulating monocytes induced by MEFs was assessed in a transwell migration assay. Circulating monocytes from 8-week-old Apoe−/− male mice were sorted (CD3−B220−NK1.1−LY6G−CD49−SIGLECF−CD45+CD11B+Ly6C+/−). Initially, MEFs were placed on the lower chamber of the transwell with DMEM 1% FBS. Sorted monocytes were placed onto the upper chamber of the transwell with RPMI 1% FBS. MEFs were stimulated with ImP 10 μM. After 24 h, the medium under transwell was collected and cells in the supernatant were quantified using LSRFortessa SORP (Becton Dickinson).
Serum cytokines measure
Blood was collected as described above. Amount of TNF and IFNγ in the serum was measured according to the manufacturer protocol using mouse TNF DuoSet ELISA (DY410) and mouse IFNγ DuoSet ELISA (DY485), respectively.
ImP treatment in grafted mice
Ldlr−/− CD45.1 mice were lethally irradiated with two doses of 550 rad (5.5 Gy), separated by at least 3 h, for a total radiation dose of 1,100 rad, followed by intravenous injection of 2 × 106 bone marrow cells from CD45.2+ Lyz2ΔRaptor or control Raptorfl/fl littermates. In parallel experiments, 2 × 106 α2-adrenoceptor cells from Rag1−/− or control C57BL/6J wild-type mice were transferred. In another set of experiments, Ldlr−/− CD45.1 mice were lethally irradiated as described above followed by intravenous injection of 2 × 106 bone marrow cells from CD45.2+ Lyz2ΔNisch or control Nischfl/fl littermates. Grafted mice were injected with 25 μg g−1 of cefovecin subcutaneously to prevent infection upon bone marrow graft. Upon reconstitution of bone marrow after 45 days, mice were treated or not with ImP (400 μg per mouse per day, Biogen Cientifica BA-F-3185.0001) for 12 weeks in the drinking water.
I1R siRNA silencing
I1R silencing in vitro was performed using Nisch siRNA (Santa Cruz sc-61202), a pool of 3 target-specific 19–25 nt siRNAs designed to knock down Nisch gene expression or control siRNA (Santa Cruz sc-36869) consisting of a scrambled sequence conjugated to 10–20 fluorescein that does not lead to the specific degradation of any cellular mRNA. Cultured MEFs were plated (2 × 105 cells per well) in a 6-well tissue culture plate in 2 ml of antibiotic-free normal growth medium supplemented with FBS. After 24 h, cells were washed with siRNA Transfection Medium (Santa Cruz sc-3686860) and siRNA duplex solution (A + B) was added after mixing by pipetting and incubating the mixed solution for 30 min at room temperature (A: 60 pmol siRNA into 100 µl siRNA Transfection Medium; B: 6 µl of siRNA Transfection Reagent (Santa Cruz sc-29528) into 100 µl siRNA Transfection Medium). MEFs were incubated with the mixed solution. After 5 h transfection, medium was removed and 2 ml of RMPI 10% FBS with antibiotics was added. After 24 h, cells were treated in vitro with and without ImP (10 μM) and co-incubated or not with AGN192403 (1 μM, I1R antagonist).
Quantitative PCR with reverse transcription
To determine Nisch expression, spleen cells were sorted as CD45+CD11b+ and CD45+CD3+ cells, and RNA extracted with the RNeasy Mini Kit and reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit with random hexamers following manufacturer’s instructions. Quantitative PCR was performed using the GoTaq qPCR Master Mix in a 7900HT Fast Real-Time PCR System. The sequence of primers used for qPCR were: Nisch sense 5′-TATGTTGTGGCACAGAAGATGG-3′; anti-sense 5′-TTCAGGCAATGGATAGTGGAT-3′; Gapdh sense 5’-TGAAGCAGGCATCTGAGGG-3′; anti-sense 5’-CGAAGGTGGAAGAGTGGGAG-3′.
Statistics
Data analysis was carried out using GraphPad Prism software v.9.0 (GraphPad Software) and Stata version 18 (StataCorp). Variables were presented as mean ± s.e.m or mean ± s.d. unless otherwise stated. The Kolmogorov–Smirnov test and Shapiro–Wilk normality test were used to determine the normal distribution of samples. Nominal variables were presented as percentages and frequencies. Comparisons between two groups were performed using two-tailed unpaired Student’s t-tests (normal distribution) or Mann–Whitney U-tests (non-normal distribution) for continuous variables or Fisher exact test for categorical variables. Unless otherwise stated, comparisons across multiple groups were assessed using one-way ANOVA (normal distribution) followed by Tukey’s post hoc test, or Kruskal–Wallis test (non-normal distribution) followed by Dunn’s test as indicated in each figure legend. Continuous variables were compared across tertiles of ImP by ANOVA or non-parametric Kruskall Wallis tests as appropriate, whereas nominal variables by applying the Cohran–Armitage test (dichotomous variables) and the Jonckheere–Terpstra test (ordinal variable) for trend. Only significant differences are indicated. For untargeted metabolomics data, P values were corrected with a Benjamini–Hochberg post hoc method (q = 0.05) and supervised (PLS-DA) analyses were applied to select the variables responsible for the separation showed by models in MetaboAnalyst (https://www.metaboanalyst.ca/MetaboAnalyst/home.xhtml)56 (Fig. 1b,c). The models were statistically validated by cross-validation tool, using the leave-1/3-out approach to exclude model overfitting.
For the human cohorts (PESA and IGT), no sample size calculation was conducted, as samples were retrospectively collected from observational studies. Instead, sample sizes were determined based on the availability of well-characterized patients with complete clinical, imaging, and biosamples from ongoing prospective observational studies. The cohorts were assembled with the goal of maximizing biological and phenotypic depth rather than targeting a predefined effect size. The inclusion of two independent cohorts with complementary phenotypes enabled replication and validation across datasets. Post hoc power considerations indicated that the sample size of the derivation cohort (PESA cohort, n = 400) was adequately powered at the 0.85 level to detect a minimum 5 nM difference in ImP levels between the group of individuals without atherosclerosis and individuals with atherosclerosis. A ratio of 1:3 between patients without and with atherosclerosis was selected to reflect the distribution of atherosclerosis in the PESA cohort, and measures of dispersion (s.d.) were derived from the group of participants without atherosclerosis in the PESA cohort. Respectively, the IGT cohort was adequately sized to detect with 0.85 power a difference of 2 nM in ImP levels between participants without and with atherosclerosis. Power considerations were based on the Mann–Whitney test for two independent groups. Type I error was prespecified at 0.05 for power calculations. Power analysis was performed with G*Power 3.1.9.6 (University of Kiel, Germany). Restricted cubic splines (natural splines, RCS) with three knots across ImP distribution were applied to produce dose–response curves and to flexibly model the potential non-linear association of continuous ImP with the main endpoints, as previously described78,79. In both cohorts, the three knots for RCS were fixed at the 10th, 50th and 90th percentile of the ImP distribution. Number of knots was selected on the basis of improvement in log likelihood among regression models with 3- or 4-knot RCS. To enhance visual clarity, plots with RCS were truncated at values exceeding the 95th percentile of the ImP distribution. We evaluated the non-linear relationships of ImP with log odds ratio of the main atherosclerosis outcomes by examining (Wald test) the non-linear terms (RCS)80. Dose–response curves in both cohorts were controlled for a set of confounders, constructed by combining LASSO regression and variables with biological plausibility. LASSO for logistic regression was used for multivariable model selection with respect to the outcome presence of atherosclerosis. Adaptive LASSO with cross-validation was employed to select the value of the LASSO penalty parameter lambda81. Adjusted differences in levels of ImP across groups of interest were estimated by multivariable linear regression analysis. To evaluate the association between ImP levels and the presence and activity of atherosclerosis within the PESA cohort multinomial logistic regression was performed and the model was adjusted on a set of confounders (age, sex, smoking status, fasting glucose, hs-CRP and haemoglobin concentration) based on previous evidence21. The proportional odds assumption was tested with the Brant Wald test. Correlation analysis of ImP, atherosclerosis traits and risk factors, gut microbiome and dietary patterns was investigated using non-parametric Spearman’s test and the resulting P values corrected with a Benjamini–Hochberg post hoc method (q = 0.05). Finally, the discriminatory capability of ImP for prevalence of subclinical or active atherosclerosis on top of traditional risk factors, including LDL-cholesterol and hs-CRP, was assessed by comparing area under the curve from corresponding receiver operating characteristic analysis. Areas under the curve were compared using the DeLong test82. Bootstrapping with 1,000 replicates was used to derive 95% bias-corrected confidence interval. All tests were two-tailed and the level of statistical significance was pre-specified at P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.