Bacterial strains and culture conditions
E. coli MG1655, UPEC CFT073, and derivative mutant strains (Supplementary Table 6) were routinely grown at 37â°C with shaking. One millilitre of culture was grown in 14-ml round-bottom culture tubes shaking at 300ârpm, or larger volumes were grown in flasks at lower shaking speed. For all liquid experiments, we used supplemented M9 medium (SM9)8 (1à M9 salts (DF0485-17, Fisher Scientific), 0.4% glucose, 2âmM MgSO4, 0.1âmM CaCl2, 2âμM ferric citrate, 3.1âgâlâ1 Neidhardt Supplement Mixture (NSM01, ForMedium) and micronutrient supplement as previously described61). Neidhardt Supplement was autoclaved for 20âmin, stirred for 10âmin, and then the other medium components were added.
Semisolid medium was prepared as previously described62 but using SM9. To prepare semisolid SM9, Neidhardt Supplement Mixture was combined with SeaPrep agarose (3.5âgâlâ1, Lonza) and autoclaved for 22âmin. Remaining medium components were added after autoclaving, as done for SM9 broth. Semisolid medium was then cooled to 37â°C. Cells were inoculated into semisolid medium at 37â°C and then briefly stirred. The resulting culture was placed in an ice bath for 30âmin to let the medium gel. The ice bath must reach higher than the liquid level of the medium to evenly chill the entire volume. The semisolid culture was carefully transferred to 37â°C.
For strain construction and plasmid preparation, E. coli were grown in LB Miller Broth (DF0446-07-5, Fisher Scientific). LB Miller plates were used for growth on solid medium unless otherwise noted. For plasmid maintenance, plates or broth were supplemented with 25âμgâmlâ1 chloramphenicol, 50âμgâmlâ1 kanamycin, or 50âμgâmlâ1 carbenicillin. Cells were routinely pelleted by centrifugation at 5,000g for 5âmin.
Plasmid construction
prmf-RFP and pmdtK-RFP were assembled by NEB HiFi assembly (E2621L) using pBbA6C-RFP63 as backbone (amplified with SB226 and SB227) and prmf-GFP64 or pmdtK-GFP64 as insert (amplified with SB224 and SB228).
All plasmids are listed in Supplementary Table 6. pBbS6C-dcas9, pBbS6C-metG, pBbS6C-metG*, and pBbS6C-yqgE were cloned by NEB HiFi assembly (E2621L) using a common vector backbone (pBbS6C-RFP63 amplified with SB167 and SB168) and the following gene inserts: pWJ445 amplified with SB170, SB171 (dCas9); MG1655 genomic DNA (gDNA) amplified with SB150, SB169 (metG); MG1655-metG* gDNA amplified with SB150, SB169 (metG*); MG1655 gDNA amplified with SB202, SB203 (yqgE).
To assemble pYqgE+ and pRFP+, intermediate plasmid pBbE6A-RFP was assembled by ligation of ZraI- and XhoI-digested pBbS6C-RFP63 and pBbE2A-RFP63. pBbE6A-RFP was amplified by PCR with SB168 and SB180 to generate the vector fragment. For pYqgE+, yqgE was amplified from MG1655 genomic DNA with SB178 and SB179. For pRFP+, RFP was amplified from pBbE2A-RFP63 with SB197 and SB198. Vector and inserts were assembled using the HiFi assembly kit (E2621L, New England Biolabs).
pBbS6A-yqgE (also called âi-pYqgE+â for âinducible pYqgE+â) was assembled by NEB HiFi assembly (E2621L) using pBbS6A-yqgE63 as backbone (amplified with SB212 and SB213) and pYqgE+ for the insert (amplified with SB180 and SB214).
Strain construction
hipA7 cells were constructed by transferring the hipA7 mutation from TH126931 to our MG1655 strain. All deletion strains were constructed using λ Red-mediated recombination65. The following primers were used to amplify the template from pKD4 for recombination: SB165 and SB166 (yqgE), SB183 and SB184 (lon), SB185 and SB186 (priA), SB191 and SB192 (sulA). For all strains except ÎpriA, the kanamycin resistance (kanr) cassette was removed with pCP20, which was subsequently lost after non-selective overnight growth at 42â°C. Strains were confirmed to have no remaining antibiotic resistance.
Removal of kanr was not successful for MG1655-ÎpriA or metG*-ÎpriA, as cultures did not grow at 42â°C. Instead, experiments in Extended Data Fig. 11a were done with the kanamycin marker still present. After outgrowths (Extended Data Fig. 11a), deletion of priA was confirmed again, and dnaC was checked for compensatory mutations66. ÎpriA strains were grown in M9 for cloning steps and then in SM9 for growth curves (Extended Data Fig. 11a).
Bioreactor growth
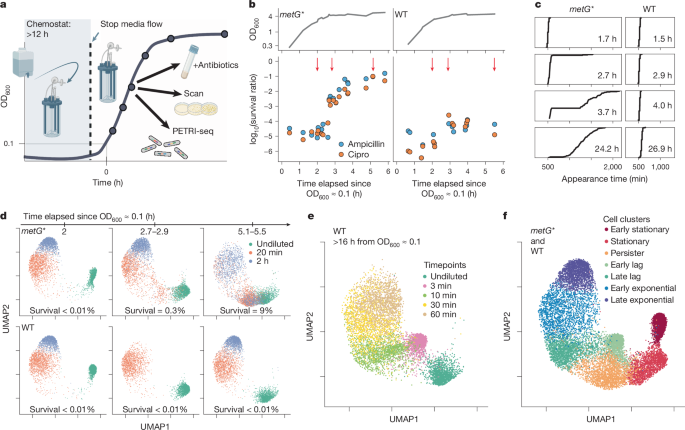
Ten litres of SM9 medium was prepared in a carboy. Around 160âml were transferred into an autoclaved vessel (DASGIP) with 500âml capacity. A silicone heater (GBH0250-1, BriskHeat) was used to bring the temperature of the medium to 37â°C, after which 100âμl of overnight culture was inoculated into the vessel, and medium flow into the vessel was turned on. An outflow pump maintained a constant level of medium. The culture was stirred with a stirrer bar at 500ârpm and air was flowed in at 0.1âlâminâ1. Medium flow rate was manually tuned to be above the E. coli doubling time. After >12âh, medium flow was turned off. Using a sampling port and syringe, samples were taken for OD600 measurement, antibiotic treatment, ScanLag, and/or PETRI-seq.
Antibiotic survival assays
To measure antibiotic tolerance, cells were incubated in SM9 containing 200âμgâmlâ1 ampicillin and/or 5âμgâmlâ1 ciprofloxacin for 4âh (unless otherwise noted) with shaking at 37â°C. For lag phase antibiotic survival, cells were taken either from the bioreactor or from an overnight culture and diluted 1:50 or 1:100 into SM9 plus antibiotics. When important, the length of the âovernightâ culture was noted (as in Fig. 5c or for 6-day stationary in Fig. 2b), but typical overnight cultures were grown for 16â24âh. For stationary phase antibiotic survival (âundilutedâ in Extended Data Fig. 1a), antibiotics were added directly to the overnight culture. To count colonies after treatment, cells were pelleted, resuspended in PBS, and then plated on LB. CFUs were counted after 48âh and compared to CFUs before antibiotic treatment. Unless otherwise noted, replicates were biological replicates from distinct single colonies picked for overnight cultures.
To assay survival âin tetâ or âafter tetâ (Fig. 3a,b), overnight cultures were diluted into fresh medium containing 54âμgâmlâ1 tetracycline. Cultures were incubated in tetracycline for 30âmin at 37â°C with shaking. Then, antibiotics were added (in tet), or cells were pelleted, washed twice in tetracycline-free fresh medium, then treated with antibiotics (after tet). Cells were kept in antibiotics for 4âh.
To assay antibiotic survival after rifampicin (Extended Data Fig. 8b), overnight cultures were diluted into fresh medium containing 200âμgâmlâ1 rifampicin. Cultures were incubated for 30âmin at 37â°C with shaking, then ampicillin or ciprofloxacin was added and incubated for 4âh (âWT in rifampicinâ). For comparison, an overnight culture was diluted into antibiotic-containing medium (without rifampicin, âWTâ on plot). To assay survival in rifampicin alone (Extended Data Fig. 8c), overnight cultures were diluted into fresh medium containing 200âμgâmlâ1 rifampicin and incubated for 1âh at 37â°C with shaking. CFUs were counted before and after rifampicin.
Appearance time assays
Cells were taken either from the bioreactor or from a standard overnight culture and ~100âCFU were spread on an LB or SM9 agar plate. Unless otherwise noted, replicates were measured from distinct single colonies picked for inoculation. To maximize reproducibility, all plates contained 25âml of medium. Colony appearance times were not different between LB and SM9 plates. As detailed previously19, plates were put on a scanner (Epson V500 Photo) in the 37â°C incubator and scanned at 15-min intervals for 24â48âh.
Scanners were controlled by ScanningManager software, and images were analysed using Matlab scripts previously published19. Appearance times were found using the appearance output of getAppearanceGrowth. Minimum colony size was set to 20 and maximum set to 100.
PETRI-seq library preparation
Growth conditions for all PETRI-seq samples are detailed in Supplementary Table 3.
PETRI-seq of E. coli cells was carried out as detailed previously2. A stepwise protocol is available at: https://tavazoielab.c2b2.columbia.edu/PETRI-seq/. In brief, cells were pelleted and fixed overnight in 4% formaldehyde. The following day, cells were washed twice in PBS with RNase inhibitor (PBS-RI) and then resuspended in 50% ethanol in PBS-RI. In 50% ethanol, cells could be stored at â20â°C for at least 2 weeks. Cells were washed twice in PBS-RI to remove the ethanol and then permeabilized with lysozyme. Cells were washed twice again and then treated with DNase. After DNase inactivation, cells were washed twice in PBS-RI. As a stopping point, cells could then be resuspended in 50% ethanol in PBS-RI and saved at â20â°C for at least 2 weeks; then they were washed twice again in PBS-RI before resuming. To continue cell preparation, the cell pellet was resuspended in PBS-RI and counted using a haemocytometer. Split-pool barcoding, cell lysis, and second strand synthesis were performed as described, yielding 20âμl purified cDNA2. For tagmentation, EZ-Tn5 (TNP92110, Biosearch Technologies) was loaded by annealing SB117 and SB118 (Supplementary Table 6), diluting the oligonucleotides to 5âμM each in 50% glycerol, and then adding 2âμl EZ-Tn5 to 8âμl of the oligonucleotides. EZ-Tn5 was incubated with the oligonucleotides for 30âmin at room temperature; loaded EZ-Tn5 was stored at â20â°C. 0.125âμl of loaded EZ-Tn5, 24.875âμl TD buffer (FC-131â1096, Illumina), and 5âμl water were added to 20âμl purified cDNA and incubated at 55â°C for 5âmin then brought to 10â°C. 12.5âμl NT (FC-131â1096, Illumina) was immediately added to stop the reaction. Tagmented cDNA was amplified in a 500âμl PCR with Q5 polymerase (M0491L, New England Biolabs): 100âμl 5à buffer, 10âμl 10âmM dNTPs (N0447L, New England Biolabs), 5âμl Q5 polymerase, 85âμl Q5 High GC Enhancer, 0.5âμM N70x (Nextera Index Kit v2 Set A, TG-131-2001, Illumina; or equivalent from Integrated DNA Technologies), 0.5âμM i50x (E7600S, New England Biolabs; or equivalent from Integrated DNA Technologies). Libraries were amplified until the early exponential phase (~16â18 cycles): 72â°C 3âmin; 95â°C 30âs; cycle: 95â°C 10âs, 55â°C 30âs, 72â°C 30âs; 72â°C 5âmin. PCR reactions were pooled (if the 500âμl reaction had been split into multiple PCR tubes), and 100âμl was taken, purified twice with AMPure XP beads (A63881, Beckman Coulter), and eluted in 30âμl water. The resulting libraries could be sequenced directly (non-depleted) or rRNA-depleted using Cas9.
rRNA depletion of PETRI-seq libraries by Cas9
PETRI-seq libraries were subjected to rRNA depletion by the canonical Cas9::crRNA::tracrRNA tripartite complex67. To prepare tracrRNA, a dsDNA template (C2425) was made by PCR of pWJ4023 with Q5 polymerase and primers W2031 and W2032. Alternatively, C2425, which is 96 bases long, could be made by ordering and annealing complementary oligonucleotides. C2425 was used for T7 in vitro transcription with the TranscriptAid T7 High Yield Transcription Kit (K0441, Thermo Scientific) by combining the following in a 20âμl reaction: 4âμl 5à reaction buffer, 2âμl 100âmM ATP, 2âμl 100âmM CTP, 2âμl 100âmM GTP, 2âμl 100âmM UTP, 1âμl T7 RNAP enzyme, 700âng C2425. The reaction was incubated at 37â°C for 4âh, during which a white precipitate became visible. 1âμl DNase I (AMPD1, Millipore Sigma) was added and incubated at 37â°C for an additional 45âmin to digest the DNA template. RNA was purified using the Norgen Biotek Total RNA purification kit (37500, Norgen) to generate J703 (tracrRNA). To prepare crRNAs, 45âμM W2034 (T7 promoter) and 45âμM W2035-W2141 (separate reaction for each) were combined in annealing buffer (10âmM Tris pH 7.5, 50âmM NaCl, 1âmM EDTA), heated to 95â°C for 5âmin then cooled to room temperature. 1âμl of annealed product was used for T7 in vitro transcription (K0441, Thermo Scientific) by adding the following: 4âμl 5à reaction buffer, 2âμl 100âmM ATP, 2âμl 100âmM CTP, 2âμl 100âmM GFP, 2âμl 100âmM UTP, 1âμl T7 RNAP enzyme, 6âμl water. The reaction was incubated at 37â°C for 4âh, during which a white precipitate became visible. One microlitre DNase I (AMPD1, Millipore Sigma) was added and incubated at 37â°C for an additional 45âmin to digest the DNA template. Each resulting crRNA was purified using the Norgen Biotek Total RNA purification kit (37500, Norgen). To anneal tracrRNA to crRNA, 70âpmol tracrRNA (J703) and 70 pmol crRNA were combined in 10âμl of annealing buffer (10âmM Tris pH 7.5, 50âmM NaCl, 1âmM EDTA), heated to 95â°C for 5âmin, then slowly cooled to room temperature to yield 7âpmolâμlâ1 tracrRNA::crRNA. All 59 annealed tracrRNA::crRNA were pooled in an equimolar ratio. rRNA was depleted by combining the following in a 50âμl reaction: 5âμl 10à reaction buffer (Z03386, GenScript), 0.74âμl tracrRNA::crRNA (5.18 pmol total tracrRNA::crRNA; 0.088 pmol of each), 10âμl Cas9 (Z03386, GenScript), 49â80âng PETRI-seq library. The reaction was incubated at 37â°C for 90âmin then purified twice with 1à AMPure beads. The library concentration was measured using the Agilent Bioanalyzer High Sensitivity DNA Kit (5067-4626, Agilent). Libraries were sequenced for 75 cycles (58 R1, 17 R2) using the NextSeq 500/550 High Output Kit v2.5 (20024906, Illumina). rRNA-depleted libraries were loaded at ~2à the recommended concentration to account for cleaved rRNA fragments without both Illumina adapters. Non-depleted libraries were loaded at ~1.5à the recommended concentration.
Our rRNA depletion strategy is in theory very similar to DASH68, which amplifies cDNA after Cas9 cleavage. Further optimization could include testing the differences between these techniques.
Fluorescence-activated cell sorting
metG* cells were transformed with fluorescent transcriptional reporters for rmf, cysK and mdtK promoters64 (Supplementary Table 6). Overnight cultures were diluted 1:100 (rmf, cysK, dual markers) or 1:50 (mdtK) into SM9 then grown for 3.5 (rmf), 3.17 (cysK), 2.5 (mdtK), 5.8 (dual cysK/rmf), or 5.25 (dual cysK/mdtK) hours at which point they reached OD600 of 0.401 (rmf), 0.336 (cysK), 0.238 (mdtK), 0.35 (dual cysK/rmf), or 0.59 (dual cysK/mdtK). Cells were centrifuged at 5,000g for 5âmin and then resuspended in PBS. Using an S3e Cell Sorter (12007058, Bio-Rad), cells were analysed, gated by forward scatter versus side scatter (Bio-Rad ProSort; Extended Data Fig. 3h,i), then sorted by GFP expression (high or low) into PBS. Sorted cells were counted (CFU), inoculated into antibiotic-containing SM9, and/or used for ScanLag. For the protein expression assay shown in Extended Data Fig. 3f,g, metG*–pcysK-GFP cells were transformed with pBbA6C-RFP63 (35290, Addgene), which expresses RFP under the LlacO1 promoter. Overnight cultures were diluted 1:50 into SM9 + 500 µM IPTG then grown for 4.6âh (OD600â=â0.182). We noted that because of the stochasticity in metG* lag times, time to reach a particular OD600 after dilution from an overnight varied substantially by experiment. Cells were resuspended in PBS, analysed, gated by forward scatter versus side scatter (Extended Data Fig. 3h,i), then sorted by GFP and RFP expression into SM9 (Extended Data Fig. 3f). GFP- or RFP-only controls were used to compensate for overlapping emissions of GFP and RFP. Because the cells come out of the sorter in PBS, the final composition of medium was 71% SM9 in PBS. Cell density was too low to successfully pellet the cells and change medium. Cells were grown at 37â°C with shaking (300ârpm) and analysed at given timepoints over the next day. OD600 stayed below 0.01 for the duration of the experiment, likely reflecting high purity of cells with long lag times and possibly reduced growth rate from 29% PBS. To see RFP expression in persister cells (Extended Data Fig. 3g), populations were gated on high GFP (cysK+). To make Extended Data Fig. 3, FlowJo 10.8.1 was used. Distributions were plotted using the layout editor. metG* cells without a fluorescent protein expression vector were used to subtract background autofluorescence (for Extended Data Fig. 3g).
Generating crRNA library with CALM
E. coli crRNA libraries were generated using CALM, as previously described3 with one minor modification. C2185 (insert library) and C2184 (backbone) were assembled by Gibson reaction (E2621L, New England Biolabs) and transformed into MG1655 cells without pWJ445 (dCas9 plasmid). The library was grown in LB broth containing 50âμgâmlâ1 kanamycin at 37â°C until OD600 reached ~0.4 (about 4âh). The resulting library was pelleted and used to miniprep an assembled crRNA plasmid library, labelled M60. Sequencing of this library confirmed high coverage of the genome with each gene targeted on average by 56 unique crRNAs.
CRISPRi screen sample collection
Supplementary Table 5 includes details about each CRISPRi library. Generally, electrocompetent cells were prepared from the parental strain containing either pWJ445 (pTet-dCas9) or pBbS6C-dCas9 (pLlacO1-dCas9). Different inducers (IPTG or aTc) were used for replicates to avoid inducer-specific effects. ~200âng of M60 (crRNA plasmid library) was electroporated with 50âμl of cells using the MicroPulser (Bio-Rad) set to the default E. coli program 1 (1âmm, 1.8âkV, 6.1âms). Cells were recovered in 500âμl SOC medium for 1.5âh at 37â°C. A small volume was taken to count colonies on LB agar with or without selection antibiotics (kanamycin + chloramphenicol) in order to calculate transformation efficiency and ensure adequate library coverage. The crRNA library contains at most 500,000 crRNAs3, and transformations routinely yielded >100 million transformants. The remaining volume of recovered cells was transferred to a flask containing 225âml SM9 with kanamycin + chloramphenicol. For YqgE/RFP overexpression screens (SBC308-SBC315; Supplementary Table 5), 500âμM IPTG was added 2âh later to induce yqgE or RFP. For all libraries, cells were grown at 37â°C until they reached an OD600 of ~0.4 (3â4âh). The resulting library (L) was divided for either late exponential/stationary dCas9 induction before lag phase assays or exponential dCas9 induction before exponential assays. Samples were also taken from L for SBC95 and SBC125 (Supplementary Table 5). Late exponential/stationary induction allowed for minimal loss of essential crRNAs (area under the curveâ=â0.48â0.52 for wild-type/metG* before and after induction), so all genes could be assayed in lag phase.
Lag phase assays
For lag phase assays, 20âml of cell library (L) was pelleted and resuspended in 20âml SM9 containing kanamycin, chloramphenicol, and either anhydrotetracycline (aTc; 20ânM) or isopropyl-β-d-thiogalactopyranoside (IPTG; 500âμM). Centrifugation likely was not necessary here but was done every time for consistency. The culture was grown overnight (14â22âh) to induce dCas9 and reach stationary phase. The next day, cells were pelleted and resuspended in the same volume of SM9 without inducer or antibiotics. Samples were taken for SBC96, SBC126, SBC191, SBC205, SBC316, SBC308, SBC310, SBC312 and SBC314 (Supplementary Table 5).
For lag outgrowth, resuspended cells were either inoculated into 500âml semisolid SM9 (~80 million cellsâper litre for SBC101, SBC131, SBC210, SBC318; ~2âÃâ109 cells per litre for SBC100, SBC130) or diluted 100à into SM9 broth (SBC102, SBC132, SBC192, SBC309, SBC311, SBC313, SBC315). Outgrowth times are shown in Supplementary Table 5.
For lag antibiotic treatment, resuspended cells were diluted 100à into SM9 containing 200âμgâmlâ1 ampicillin and 5âμgâmlâ1 ciprofloxacin and incubated for the indicated amount of time (Supplementary Table 5). After antibiotic treatment, cells were pelleted, washed in PBS, then resuspended in SM9 and inoculated into 500âml semisolid SM9 medium at a density ~50âÃâ106 cells per litre. After 2 days, cell samples were collected for SBC98, SBC99, SBC128, SBC129, SBC207, SBC208, SBC209 and SBC317. Semisolid medium was used to minimize interclone competition.
Exponential assays
For exponential assays, dCas9 was induced in exponential phase, and cells were not grown overnight to stationary phase. The cell library (L) was diluted 200x into SM9 containing kanamycin, chloramphenicol, and 20ânM aTc or 500âμM IPTG. Cells were grown for 3.5â4.5âh (OD600 = ~0.2-0.4). Samples were taken for SBC133 and SBC211 (Supplementary Table 5) and also diluted 100à into SM9 containing kanamycin, chloramphenicol, and 20ânM aTc or 500âμM IPTG. These cells were grown for 3â4âh then sampled for SBC134 and SBC212.
CRISPRi library preparation
Collected cell samples (described above) were pelleted and miniprepped (Qiagen). 400âng of DNA were amplified in a 60âμl PCR with Q5 polymerase (M0491L, New England Biolabs), 0.5âμM of forward primer (equimolar mixture of W1397, W1398, W1399, W1400), and 0.5âμM of reverse primer (W1699). The reaction was thermocycled as follows: 98â°C 30âs; 10à 98â°C 10âs, 55â°C 20âs, 72â°C 30âs; 72â°C 2âmin. PCR products were purified by double-sided AMPure cleanup (left-side ratio = 0.8Ã; right-side ratio = 1.4Ã) then eluted in 40âμl H2O. 2.5âμl of purified DNA was used for a second PCR in 100âμl using Q5 polymerase, 0.5âμM forward primer (CRISPRi_PCR_2_F; Supplementary Table 6), and 0.5âμM reverse primer (CRISPRi_PCR_2_R; Supplementary Table 6). The reaction was thermocycled as follows: 98â°C 30âs; 6à 98â°C 10âs, 55â°C 20âs, 72â°C 30âs; 72â°C 2âmin. PCR products were purified by two AMPure cleanups (first 0.9Ã, then 0.8Ã) and eluted in 30âμl. The library concentration was measured using the Agilent Bioanalyzer High Sensitivity DNA Kit (5067-4626, Agilent). Libraries were sequenced for 75 cycles using the NextSeq 500/550 High Output Kit v2.5 (20024906, Illumina). Single-end reads are ideal because only Read 1 is useful for mapping crRNAs. However, depending on the forward primer used for PCR 2, as few as 58 cycles can be allocated to read 1.
Antibiotic susceptibility with bortezomib
Bortezomib (5043140001, Millipore Sigma) stock was prepared by dissolving in DMSO. For the assays in Extended Data Fig. 11d,e, single colonies were picked into SM9 containing 1% DMSO and 100âμM bortezomib. Control (â bzmb) cultures were started in the same way but SM9 contained 1% DMSO and no bortezomib. After overnight culture, antibiotic survival was assayed as described in âAntibiotic survival assaysâ, but for lag phase assays, antibiotic-containing SM9 was supplemented with 1% DMSOâ±â100âμM bortezomib.
Lag times after bortezomib treatment
For full growth with bortezomib (dotted black line in Fig. 5b), single colonies of metG*-ÎsulA cells were picked into 1âml SM9 containing 1% DMSO and 100âμM bortezomib (5043140001, Millipore Sigma). For bortezomib addition during stationary phase (dotted pink line in Fig. 5b), single colonies of metG*-ÎsulA cells were picked into 1âml SM9. After 20âh, bortezomib was added to 100âμM. For bortezomib treatment during lag phase (dotted green line in Fig. 5b) or not at all (control; grey line in Fig. 5b), single colonies of metG*-ÎsulA cells were picked into 1âml SM9 containing 1% DMSO. After 24âh of growth, all cultures were diluted 100à into SM9 containing either 100âμM bortezomib and DMSO (green line in Fig. 5b) or only 1% DMSO (all other samples). Cells were grown on a plate reader (37â°C with continuous shaking; PowerWave XS2, BioTek) and OD600 measured at 10-min intervals.
Stationary phase translation assay
E. coli cells of the indicated genotype (Fig. 5f) containing pBbS6C-RFP were grown for 24âh in 1âml SM9. Two-hundred microlitres of overnight culture were transferred to a 96-well plate and supplemented with 2.5âmM IPTG. Cells were grown on a plate reader (37â°C with continuous shaking; Synergy Neo2, BioTek). RFP (570 excitation, 620 emission) and OD600 were measured at 10-min intervals.
metG complementation of metG* mutation
Wild-type or metG* cells carrying either pBbS6C-metG or pBbS6C-metG* (Supplementary Table 6) were grown overnight in 1âml SM9 containing chloramphenicol. Overnight cultures were diluted 1,000à into SM9 containing chloramphenicol and 500âμM IPTG. These cultures were grown overnight again. The following day, lag phase antibiotic survival was assayed with ampicillin and ciprofloxacin (Extended Data Fig. 9d).
Quantitative proteomics
Overnight cultures of 3 colonies each of MG1655, metG*, and metG*-Îlon-ÎsulA were grown in 1âml SM9 for ~19âh at 37â°C. Stationary samples were taken directly from the overnight cultures. For wild-type (MG1655) exponential cells (Extended Data Fig. 7), MG1655 overnight cultures were diluted 200à into fresh SM9 and grown for 90âmin (final OD600â=â0.2â0.23). For metG* lag/persister cells (Extended Data Fig. 7), metG* overnight cultures were diluted 100à into fresh SM9 and grown for 30âmin. OD600 did not increase in that 30âmin (replicate 1: ODinitialâ= 0.069, OD30minâ=â0.065; replicate 2: ODinitialâ=â0.07, OD30minâ=â0.065; replicate 3: ODinitialâ=â0.072, OD30minâ=â0.066).
Cells were collected in Eppendorf tubes and washed twice with ice-cold PBS. Cells were then lysed in lysis buffer containing 8âM urea, 0.1âM ammonium bicarbonate, and protease inhibitors (1 mini-Complete EDTA-free tablet). The lysate was cleared by centrifugation at 14,000ârpm for 30âmin at 4â°C. The supernatant was transferred to a new tube, and the protein concentration was determined using a BCA assay (Pierce). Subsequently, 10âµg of total protein was subjected to disulfide bond reduction with 10âmM DTT (at 56â°C for 30âmin) followed by alkylation with 10âmM iodoacetamide (at room temperature for 30âmin in the dark). Excess iodoacetamide was quenched with 5âmM DTT (at room temperature for 15âmin in the dark). Samples were then diluted sixfold with 50âmM ammonium bicarbonate and digested overnight at 37â°C with a trypsin/Lys-C mix (1:100). The next day, digestion was stopped by the addition of 1% TFA (final v/v), followed by centrifugation at 14,000g for 10âmin at room temperature to pellet precipitated lipids. Cleared digested peptides were desalted on an SDB-RPS Stage-Tip disk69 and dried down in a speed-vac. Peptides were resuspended in 10âµL of 3% acetonitrile/0.1% formic acid and injected onto a Thermo Scientific Orbitrap Fusion Tribrid mass spectrometer using a DIA method for peptide MS/MS analysis.
The UltiMate 3000 UHPLC system coupled with an EASY-Spray PepMap RSLC C18 column was used to separate fractionated peptides with a gradient of 5â30% acetonitrile in 0.1% formic acid over 90âmin at a flow rate of 300ânlâminâ1. After each gradient, the column was washed with 90% buffer B for 10âmin and re-equilibrated with 98% buffer A (0.1% formic acid, 100% HPLC-grade water) for 30âmin. Survey scans of peptide precursors were performed from 350â1,200âm/z at 120âK FWHM resolution with a 1âÃâ106 ion count target and a maximum injection time of 60âms. The instrument was set to run in top speed mode with 3-s cycles for the survey and MS/MS scans. After a survey scan, 26âm/z DIA segments were acquired from 200â2,000âm/z at 60âK FWHM resolution with a 1âÃâ106 ion count target and a maximum injection time of 118âms. HCD fragmentation was applied with 27% collision energy, and resulting fragments were detected using the rapid scan rate in the Orbitrap. The spectra were recorded in profile mode.
DIA data were analysed with the MaxDIA software platform within the MaxQuant software environment using a library-free approach70. The search was set up with the reference E. coli proteome database downloaded from UniProt. The false discovery rate (FDR) was set to 1% at the peptide precursor level and 1% at the protein level. Results obtained from MaxQuant were further analysed using the standard pipeline for differential analysis with the DEP package71. Proteins were filtered for inclusion in 2 out of 3 replicates of at least one condition. Data was normalized by variance stabilizing transformation. Missing data was imputed using the MinProb method with qâ=â0.01. Significantly enriched proteins were defined by alphaâ=â0.05 and lfcâ=âlog2(1.5) (Supplementary Table 2). For the principal components analysis (PCA) in Extended Data Fig. 7c, LFQ intensity (for included samples) was log-transformed and scaled with StandardScaler to centre each protein with mean of 0 and s.d. of 1. Principal components were calculated from all proteins using sklearn72. See next section for PCA and UMAP in Extended Data Fig. 7a,b.
PETRI-seq analysis
Barcode demultiplexing was carried out as previously described2 with the following minor modification73: before extracting the unique molecular identifier (UMI) sequence, PEAR74 was used to merge reads 1 and 2 when they overlapped. Only non-overlapping reads were carried forward because read 2 should contain cDNA sequence, and the end of read 1 should contain barcode 1. Note that this may not apply when sequencing more than 75 cycles. Also, read 2 was trimmed if it matched the reverse complement of the end of read 1, an artefact we think occurs due to hairpin formation. The full pipeline uses trimmomatic75 (v0.33) to filter reads, Cutadapt76 (v1.18) to demultiplex, UMI-tools77 (v0.5.5) to extract UMIs, bwa78 (v0.7.17) to align, and featureCounts79 (v1.6.3) to annotate features.
Seurat (version 4.1.1)80 was used for normalization, dimensionality reduction, and clustering of PETRI-seq data. In brief, the matrices produced by demultiplexing and UMI collapsing were read into a Seurat object. All MG1655 samples in this study (Supplementary Table 3) were combined in the same Seurat object. For Extended Data Fig. 6gâj, a new Seurat object was made with all MG1655 cells plus CFT073 cells; accessory genes only in the CFT073 genome were omitted. For all analysis, rRNA counts were excluded except for Extended Data Fig. 8. Barcodes were filtered for more than 9 and fewer than 1,000 mRNA UMIs. All cells were then downsampled to 38 UMIs using the SampleUMI function (max.umiâ=â38, upsampleâ=âFALSE). UMI counts were log-normalized using the geometric mean of all cell UMI counts as a scale factor. Gene counts were scaled and centred to a mean of 0 and s.d. of 1 (Seurat ScaleData). Principal components were calculated with all genes. For the full cell atlas (Fig. 1f), principal components 1â10 were used to compute UMAP81 coordinates (default parameters) and to find neighbouring cells. Clusters were found using default parameters82 (Louvain algorithm) at resolution 0.32. For hipA7 cells alone (Extended Data Fig. 5a), principal components 1â5 were used to find neighbouring cells, and clusters were found at resolution 0.1. For extended stationary (6-day) wild-type cells with metG* and standard wild-type cells (Extended Data Fig. 5f), principal components 1â6 were used to find neighbouring cells, and clusters were found at resolution 0.16. For the full atlas downsampled to ~30 mRNA UMIs (Extended Data Fig. 5i), cells were downsampled as described with max.umiâ=â30. Then, only cells with exactly 29 or 30 mRNA UMIs were kept in the Seurat object. Cells were processed and clustered as with the full atlas (10 principal components, resolutionâ=â0.34). For CFT073 cells alone (Extended Data Fig. 6d,e), CFT073 accessory genes were included, principal components 1â10 were used to find neighbouring cells, and clusters were found at resolution 0.31. For CFT073 with MG1655 cells (Extended Data Fig. 6g), principal components 1â10 were used to find neighbouring cells, and clusters were found at resolution 0.38.
To project proteomics samples with scRNA-seq (Extended Data Fig. 7a,b), proteomics samples were log-normalized using the geometric mean of the scRNA-seq library. Proteomics samples were then merged into a single Seurat object with downsampled, log-normalized scRNA-seq data. This entire Seurat object was scaled and centred with ScaleData. For the PCA (Extended Data Fig. 7a), loadings were extracted from the scRNA-seq Seurat object and used to project all cells and proteomic samples. For UMAP (Extended Data Fig. 7b) and clustering (Extended Data Fig. 7a,b), principal components 1â6 were used with n.neighborsâ=â50 and k.paramâ=â50. Clusters were found at resolution 0.32. If principal components 1â10 and default n.neighbors and k.param are used (as with scRNA-seq alone), then the stationary and lag proteomes form their own cluster; exponential proteomes still cluster with early exponential transcriptomes.
When the full cell atlas is shown or analysed (Fig. 1 and Extended Data Figs. 1nâu and 5i), only cell samples relevant up to that point in the text are shown or included in expression analysis, but all cells (as listed in Supplementary Table 3) were used to compute principal components, UMAP coordinates, and cell clusters. See Supplementary Table 3 for details of which cell samples are included in each figure.
For Extended Data Fig. 8, which defines transcriptional deficiency, different thresholds were used to retain cells with very low mRNA counts. Specifically, in Extended Data Fig. 8f,g, all cells with total RNA above a library-specific threshold (between 16â64 total UMIs) were retained. By contrast, Fig. 2f includes only cells with at least 10 mRNAs, as these are the cells used for UMAP and clustering. rRNA depletion is also important to consider when defining transcriptional deficiency (Extended Data Fig. 8dâf). Extended Data Fig. 8d,e only shows libraries that were not subjected to rRNA depletion. In Extended Data Fig. 8f, all libraries are included with a slightly different threshold used for depleted or non-depleted libraries.
Differential expression analysis from scRNA-seq
To find genes differentially expressed between cell clusters or pre-defined populations, a custom pipeline combining edgeR83 and Seuratâs FindMarkers tool was used. EdgeR was used with TMM normalization to calculate log2(fold change) from pseudobulk samples. Pseudobulk samples are calculated by summing all counts from all single cells of a given population; single-cell transcriptomes are taken before downsampling. For P values, limmaâs84 rankSumTestWithCorrelation (the default for Seuratâs FindMarkers; two-sided WilcoxonâMannâWhitney) was used with downsampled, log-transformed single-cell data as input. Using downsampled cells for significance testing gives the result most consistent with the centred edgeR data. Total UMI counts by sample (before and after downsampling) are provided in Supplementary Table 4.
CRISPRi analysis
CRISPRi sequencing reads were aligned to reference genomes for E. coli then to S. aureus (used for library manufacturing3). Functional spacers were identified as described3 based on presence of an âNGGâ PAM sequence. Only functional E. coli spacers were used for downstream analysis.
For lag and exponential comparisons, spacer abundance post-outgrowth was compared to pre-outgrowth. For lag antibiotic treatment, spacer abundance post-antibiotics plus outgrowth was compared to after outgrowth only. For simplicity, consider post-antibiotics as a âpostâ condition relative to outgrowth only (âpreâ) in the description below.
To compare CRISPRi libraries, spacers were filtered to remove any position with fewer than 10 reads in both post and pre libraries. Then, the frequency of each spacer was calculated by dividing the number of reads for that spacer by the total number of reads in the library. A pseudocount of 0.99 was added to spacers with 0 counts. Based on the assumption that spacers targeting intergenic regions outside of promoters would not affect phenotypes, we used these intergenic spacers to normalize spacer abundance in both pre and post libraries. All spacer frequencies were normalized as follows (for exemplified spacer labelled A):
$${{\rm{enrichment}}}_{{\rm{A}}}={\log }_{2}{({{\rm{spacer}}}_{{\rm{A}}}/{{\rm{GM}}}_{{\rm{null}}})}_{{\rm{post}}}-{\log }_{2}{({{\rm{spacer}}}_{{\rm{A}}}/{{\rm{GM}}}_{{\rm{null}}})}_{{\rm{pre}}}$$
where GMnull is the geometric mean of the frequencies of all null (intergenic) spacers.
To calculate gene enrichment scores, mean enrichment scores for spacers aligned within or directly upstream of each gene were calculated. The number of spacers mapping to each gene varied, which was important for computing gene enrichment P values. The null distribution of enrichment scores for intergenic spacers was randomly sampled to generate pseudogenes with n spacers. This was repeated to generate 100,000 simulated replicates for every relevant n. To assign a P value, each gene enrichment score was compared to a simulated null distribution with the same number of spacers as included for that gene. Significantly enriched or depleted genes were found based on a FDR of 0.1 using the BenjaminiâHochberg method85. In most cases, significant genes were further filtered by significance in multiple replicates. For hipA7, only one replicate of each screen was done. In Fig. 4b, we wanted to highlight top hits, so we used Bonferroni correction to threshold only the most significant hits. In other figures, we used FDR of 0.1 for the hipA7 replicates.
For each gene in the CRISPRi screens, enrichment and significance were calculated independently for crRNAs targeting the antisense or sense strand; the strand with strongest effect (by significance then enrichment score) is determined and included in each relevant figure. For Fig. 4b,c, strand is noted in source data. In Fig. 4d and Extended Data Fig. 10, the strand shown is antisense unless otherwise noted. To assess depletion of essential genes (Extended Data Fig. 9f,g), we used a stringent set of genes found to be essential in all of four previous datasets86.
Pathway enrichment with iPAGE
To find pathways significantly correlated with principal component 1 or 2 of the cell atlas (Extended Data Fig. 1o), we divided the principal component loadings into high (greater than 0.025) and low (less than â0.025) groups. We ran iPAGE87 in discrete mode (up, down) with maximum P value of 0.001 and independenceâ=â0. Redundant pathways were filtered manually, and representative ones are shown.
To find genes enriched in either the early lag or the persister cluster (Extended Data Figs. 1pâu and 5k,l), differential expression analysis was performed as described. Using the BenjaminiâHochberg method85, an FDR of 0.01 was applied to select significantly over- and under-represented genes. These significance scores were used as input for iPAGE87, which was run in discrete mode (up, down, neutral) with maximum P value of 0.05 and independenceâ=â0. Pathways were then filtered further by P values indicated in figure legends. Redundant pathways and those indicating enrichment of a single operon were filtered manually; representative terms are shown. To compute mean expression, the AverageExpression function in Seurat was used, and mean gene expression values were averaged for all genes in a given set.
To find genes enriched in each persister type versus early exponential cells (Extended Data Figs. 2 and 6k) differential expression analysis was performed as described. Using the BenjaminiâHochberg method85, an FDR of 0.01 was applied to select significantly over- and under-represented genes. These significance scores were used as input for iPAGE87, which was run in discrete mode (up, down, neutral) with maximum P value of 0.1 and independenceâ=â0. Pathways shown in Extended Data Fig. 2aâc(iv) are the top (Pâ<â0.0005) non-redundant gene sets overexpressed in each persister type; when these pathways are also significant for another persister type, they are also labelled in that panel (*Pâ<â0.05, **Pâ<â0.005, ***Pâ<â0.0005).
To find genes enriched in persister cell groups versus tetracycline-treated cells (Figs. 3c and 4e and Extended Data Figs. 9a,b,e,iân and 10a,b), differential expression analysis was performed as described. An FDR of 0.05 was applied85 to select over- and under-represented genes. These significance scores were used as input for iPAGE87, which was run in discrete mode (up, down, neutral) with maximum P value of 0.05 and independenceâ=â0. To select pathways to show in Fig. 3c and Extended Data Fig. 9b, loadings of principal component 1 were also used as input for iPAGE (continuous mode, 8 bins, max_pâ=â0.005, independenceâ=â0). The intersection of gene sets enriched in the top PC1 bin (Pâ<â0.001) and gene sets over-represented in at least one persister type (Pâ<â0.05; based on marker genes versus tetracycline-treated cells) are shown in Fig. 3c and Extended Data Fig. 9b. Redundant pathways were manually filtered.
To find genes enriched after CRISPRi perturbation (Extended Data Fig. 10câe), an FDR of 0.1 was applied85 using P values (computed as described above from null distribution) to select over- and under-represented genes. These significance scores were used as input for iPAGE87, which was run in discrete mode (up, down, neutral) with maximum P value of 0.05 and independence = 0. Gene sets were subsequently filtered by P valueâ<â0.01. For each gene, antisense- and/or sense-targeting crRNAs can be significant. For this analysis, only one strand was used for input; if one or both were significant in a single direction, the gene was assigned that direction, but if the antisense and sense cRNAs were significant in opposite directions, then the one with the higher enrichment score (absolute value) was used. Pathways significantly enriched in >3 of 5 metG* replicates, >1 of 3 wild-type replicates, or in 1 hipA7 replicate are shown in Extended Data Fig. 10câe.
To find pathways enriched in proteomics data (Extended Data Fig. 12aâd), differential protein analysis was performed as described with DEP. Fold changes were used as input for iPAGE87, which was run in continuous mode with 5 bins and maximum P value of 0.05.
Identification of E. coli gene homologues
The proteins of E. coli K12 (nâ=â4,136) were downloaded from Biocyc88 version 25.1. To identify potential homologues, E. coli proteins were searched against genomes of diverse microbial organisms. A total of 2,421 genomes downloaded from JGI IMG were included in the search89. These genomes were selected based on quality (High Qualityâ=ââYesâ in IMG portal) and optimization for biodiversity. They represent 39 phylum, 68 classes and 168 orders (Supplementary Table 7) based on GTDB taxonomic classification90. The protein search was done using DIAMOND91 under specific parameters: âblastp -e 1e-10 -k 10000000 –query-cover 66 –subject-cover 50 -b8 -c1â. Protein hits with maximum e-value equal to E-10 were kept as potential homologues for downstream analysis. For each protein, the number of genomes with homologues was counted and converted to frequency, shown in Extended Data Fig. 11q.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.