Characterization and maintenance of hiPS cells
The hiPS cell lines used in this study were validated using methods described in previous studies. Genome-wide single-nucleotide polymorphisms (SNP) genotyping was performed using the Illumina genome-wide GSAMD-24v2-0 SNP microarray at the Children’s Hospital of Philadelphia. Cultures were tested for and maintained Mycoplasma free. A total of five control hiPS cell lines derived from fibroblasts collected from five healthy participants were used for experiments13 (Supplementary Table 1). Two of the control hiPS cell lines were used to generate SCN9A KO hiPS cell lines, and one control hiPS cell line was used to generate the SCN9A gain-of-function hiPS cell line. Approval for experiments was obtained from the Institutional Review Board panel of Stanford University. Informed consent was obtained from all participants.
Human primary tissue
Human brain tissue samples were obtained under a protocol approved by the Research Compliance Office of Stanford University. PCW15 tissue was delivered on ice, and DRG were dissected immediately after arrival.
Generation of organoids (hCO, hDiO, hdSpO and hSeO) from hiPS cells
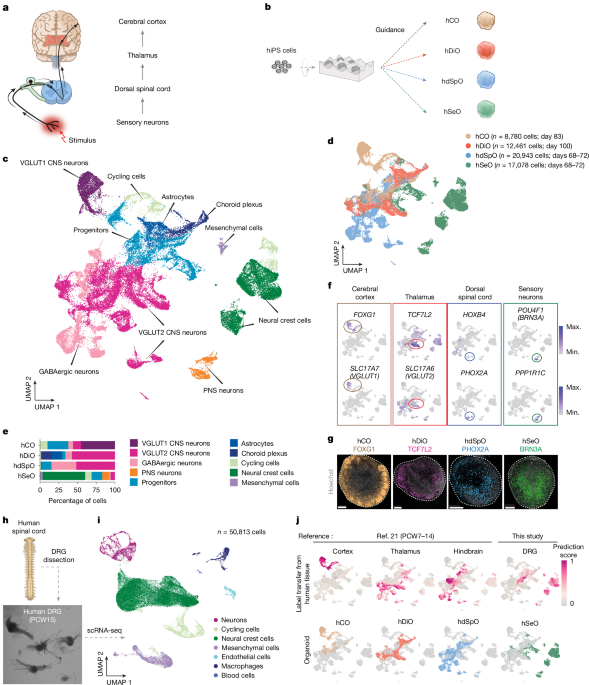
For neural differentiation, hiPS cells were cultured on vitronectin-coated plates (A14700; Life Technologies) in Essential 8 Medium (A1517001; Life Technologies). Cells were passaged every 4–5 days with UltraPure 0.5 mM EDTA, pH 8.0 (15575; Thermo Fisher Scientific). For the generation of regionalized neural organoids, hiPS cells were incubated with Accutase (AT104; Innovative Cell Technologies) at 37 °C for 7 min and dissociated into single cells. Optionally, 1–2 days before organoid formation, hiPS cells were exposed to 1% dimethylsulfoxide (472301; Sigma-Aldrich) in Essential 8 Medium. For aggregation into organoids, about 3 × 106 single hiPS cells were seeded per AggreWell 800 plate well in Essential 8 Medium supplemented with the Rho-associated coiled-coil containing kinases (ROCK) inhibitor Y27632 (10 µM; S1049; Selleckchem), centrifuged at 100g for 3 min and then incubated at 37 °C in 5% CO2. On the next day (day 1), organoids consisting of approximately 10,000 cells were collected and transferred into ultra-low attachment plastic dishes (3262; Corning) in Essential 6 Medium (A1516401; Thermo Fisher Scientific) supplemented with patterning molecules, as shown in Extended Data Fig. 1.
The hCO were generated as described previously10,55. For days 1–6, Essential 6 Medium was changed every day and supplemented with dorsomorphin (2.5 µM; P5499; Sigma-Aldrich) and SB-431542 (10 µM; 1614; R&D Systems), and was changed every day. On day 7, the organoids were transferred to neural medium containing Neurobasal-A Medium (10888022; Thermo Fisher Scientific), B-27 Supplement, minus vitamin A (12587010; Thermo Fisher Scientific), GlutaMAX Supplement (1:100; 35050079; Thermo Fisher Scientific), penicillin–streptomycin (1:100; 15070063; Thermo Fisher Scientific), supplemented with FGF2 (20 ng ml−1; 233-FB; R&D Systems) and EGF (20 ng ml−1; 236-EG; R&D Systems) until day 22. From days 23 to 46, the neural medium was supplemented with BDNF (20 ng ml−1; 450-02; PeproTech), NT3 (20 ng ml−1; 450-03; PeproTech), l-ascorbic acid 2-phosphate trisodium salt (200 µM; 323-44822; Wako), N6, 2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (cAMP; 50 μM; D0627; MilliporeSigma) and cis-4, 7, 10, 13, 16, 19-docosahexaenoic acid (10 μM; D2534; MilliporeSigma). From day 47, a neural medium containing Neurobasal-A Medium was used for changes in the medium (every 4 days).
The hDiO were generated as described previously11. For days 1–6, Essential 6 Medium was changed every day and supplemented with dorsomorphin and SB-431542. On day 5, the medium was additionally supplemented with 1 μM CHIR (S1263; Selleckchem). On day 7, organoids were maintained in a neural medium supplemented with CHIR. On day 9 of differentiation, the neural medium was supplemented with 100 nM SAG (days 9–15; 566660-1MG; MilliporeSigma), in addition to the CHIR. Furthermore, on days 12–18 of differentiation, organoids were supplemented with 30 ng ml−1 of BMP7 (120-03P; PeproTech), in addition to the compounds described above. From days 19 to 46, the neural medium was supplemented with BDNF, NT3, l-ascorbic acid 2-phosphate trisodium salt, cAMP and cis-4, 7, 10, 13, 16, 19-docosahexaenoic acid. For days 19–25, DAPT (2.5 μM, 72082; STEMCELL Technologies) was added. From day 47, a neural medium containing Neurobasal-A Medium was used for changes in the medium (every 4 days).
To generate hdSpO, from days 1 to 6, organoids were maintained in Essential 6 Medium supplemented with SB-431542 and dorsomorphin. From days 5 to 6, CHIR (3 μM) was added. On day 7, organoids were transferred to a neural medium supplemented with EGF, retinoic acid (0.1 μM; R2625; Sigma-Aldrich) and CHIR (3 μM; S1263; Selleckchem). On day 22, the medium was supplemented with BDNF, cAMP, l-ascorbic acid 2-phosphate trisodium salt, insulin-like growth factor 1 (IGF-1) (10 ng ml−1; 100-11; PeproTech) until the end of experiments. From days 22 to 28, DAPT was added.
To generate hSeO, from days 1 to 6 in suspension, organoids were maintained in Essential 6 Medium supplemented with SB-431542. From days 1 to 4, BMP4 (5 ng ml; 120-05ET; PeproTech) was added. From days 3 to 5, CHIR (600 nM) was added. On day 6 in suspension, the organoids were transferred to a neural medium supplemented with SB-431542. From day 9, BDNF, GDNF (25 ng ml−1; 450-10; PeproTech) and NGF (25 ng ml−1; 450-01; PeproTech) were supplemented until the end of the experiment. From days 9 to 14, the medium was supplemented with SB-431542. From days 15 to 20, DAPT was included.
Cryosection and immunocytochemistry
Organoids and assembloids were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) overnight at 4 °C. They were then washed in PBS and transferred to 30% sucrose/PBS for 2–3 days until the organoids/assembloids sank into the solution. Subsequently, they were rinsed in optimal cutting temperature (OCT) compound (Tissue-Tek OCT Compound 4583; Sakura Finetek) and 30% sucrose/PBS (1:1), embedded and snap-frozen using dry ice. For immunofluorescence staining, 30-µm-thick sections were cut using a Leica CM1860 cryostat. Cryosections were washed with PBS to remove excess OCT compound from the sections and blocked in 10% normal donkey serum (NDS, S30-100ML; MilliporeSigma), 0.3% Triton X-100 (T9284-100ML; MilliporeSigma) and 1% BSA diluted in PBS for 1 h at room temperature. The sections were then incubated overnight at 4 °C with primary antibodies diluted in PBS containing 2% NDS and 0.1% Triton X-100. PBS was used to wash the primary antibodies, and the cryosections were incubated with secondary antibodies in PBS with the PBS containing 2% NDS and 0.1% Triton X-100 for 1 h. The following primary antibodies were used for staining: anti-FOXG1 (rabbit; M227; 1:400 dilution; Takara), anti-TCF7L2 (rabbit; 2569S; 1:200 dilution; Cell Signaling Technology), anti-PHOX2A (rabbit; ab155084; 1:200 dilution; Abcam), anti-BRN3A (mouse; MAB1585; 1:200 dilution; Sigma), anti-mCherry (goat; orb153320; 1:1,000 dilution; Biorbyt), anti-NK1R (rabbit; S8305; 1:200 dilution; Sigma), anti-VGLUT2 (mouse; MAB5504; 1:200 dilution; Millipore), anti-NF-H (mouse; NE1023; 1:200 dilution; Millipore) and anti-PAX2 (goat; AF3364; 1:200 dilution; R&D). The following secondary antibodies were used for staining: Alexa Fluor 647 AffiniPure donkey anti-rabbit IgG (H&L) (711-605-152; 1:1,000 dilution; Jackson ImmunoResearch), Alexa Fluor 568 donkey anti-goat IgG (H&L) (A11057; 1:1,000 dilution; Thermo Fisher Scientific), Alexa Fluor 568 donkey anti-rabbit IgG (H&L) (A10042; 1:200 dilution; Thermo Fisher Scientific), Alexa Fluor 647 donkey anti-mouse IgG (H&L) (A31571; 1:200 dilution; Thermo Fisher Scientific) and Alexa Fluor 568 donkey anti-mouse IgG (H&L) (A10037; 1:1,000 dilution; Thermo Fisher Scientific). Nuclei were visualized with the Hoechst 33258 dye (H3549; 1:10,000 dilution; Life Technologies). Cryosections were mounted for microscopy on glass slides using Aqua-Poly/Mount (18606; Polysciences) or VECTASHIELD (H-1000; Vector Laboratories) and imaged on a confocal microscope. Images were processed and analysed using Fiji (v.2.14.0) and Imaris (Oxford Instruments).
Real-time qPCR
Three to five organoids were collected in the same tube and considered as one sample. The RNA from samples was isolated using the RNeasy Plus Mini kit (74136; QIAGEN). Template cDNA was prepared by reverse transcription using the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (11752250; Thermo Fisher Scientific). qPCR was performed using the SYBR Green PCR Master Mix (4312704; Thermo Fisher Scientific) on a QuantStudio 6 Flex Real-Time PCR System (4485689; Thermo Fisher Scientific). The primers used in this study are listed in Supplementary Table 2.
Preparation of scRNA-seq library and data analysis
Dissociation of organoids, assembloids and human DRG was performed, as described previously10,49,56,57. For the organoid dissociation, four to five randomly selected organoids were pooled to obtain a single-cell suspension. For assembloids, one to two assembloids were pooled for dissociation. To match the relative cell numbers across different regions, one to two organoids from each of the four regions, totalling four to eight organoids, were pooled for dissociation only when comparing organoids to assembloids. For human primary tissue, one to two DRG were pooled for dissociation. Samples were incubated in 30 U ml−1 of papain enzyme solution (LS003126, Worthington Biochemical) and 0.4% DNase (12,500 U ml−1; LS2007, Worthington Biochemical) at 37 °C for 45 min. After enzymatic dissociation, organoids were washed with a solution containing protease inhibitor and gently triturated to obtain a single-cell suspension. Cells were resuspended in 0.04% BSA/PBS (B6917-25MG, MilliporeSigma) and filtered through a 70-μm Flowmi Cell Strainer (H13680-0070, Bel-Art), and the number of cells were counted. To target 7,000 cells after recovery, approximately 11,600 were loaded per lane on a Chromium Single Cell 3′ chip (Chromium Next GEM Chip G Single Cell Kit; PN-1000127, 10x Genomics), and cDNA libraries were generated with the Chromium Next GEM Single Cell 3′ GEM, Library & Gel Bead Kit v.3.1 (PN-1000128 and PN-1000269, 10x Genomics) according to the manufacturer’s instructions. Each library was sequenced using the Illumina NovaSeq S4 2 × 150 bp (Admera Health) and NovaSeq X 28 + 90 bp (CZ Biohub). Unique molecular identifier (UMI) counting was performed using the ‘count’ function (–include-introns=TRUE) in Cell Ranger (v.7.0.1 and 7.1.0 for organoids; v.7.2.0 for the human DRG and organoid versus assembloid comparison) with GRCh38/Ensembl 98 reference (available at https://cf.10xgenomics.com/supp/cell-exp/refdata-gex-GRCh38-2020-A.tar.gz). Further downstream analyses were performed using the R package Seurat (v.4.3.0). Genes on the X or Y chromosome were removed from the count matrix to avoid biases in clustering due to the sex of the hiPS cell lines.
For comparison across four region organoids, hSeO samples (n = 4) and hdSpO samples (n = 5) from this study, along with hDiO samples11 (n = 3; GSE224766) and hCO samples13 (n = 2; GSE145122), were aggregated using the ‘aggr’ function (–normalize=mapped) in Cell Ranger. Cells with more than 7,500 or less than 2,000 detected genes, with less than 2,000 UMI counts or with mitochondrial content higher than 15% were excluded. Genes that were not expressed in at least three cells were not included in the analysis. Gene expression was normalized using a global-scaling normalization method (normalization method; ‘LogNormalize’; scale factor 10,000), and the 2,000 most variable genes were selected (selection method; ‘vst’) and scaled (mean = 0 and variance = 1 for each gene). The top 50 principal components (PCs) were used for clustering (resolution of 1.0) using the ‘FindNeighbors’ and ‘FindClusters’ functions and for visualization with UMAP. Clusters were annotated based on the expression of known marker genes.
For the analysis of hSeO and hdSpO, cells with more than 10,000 or less than 2,500 detected genes, with less than 2,000 UMI counts or cells with mitochondrial content higher than 15% were excluded. Genes that were not expressed in at least three cells in each library were not included. Normalization and variable gene detection were performed, as described above. The hSeO samples (n = 4) and hdSpO samples (n = 5) were each separately integrated using ‘FindIntegrationAnchors’ and ‘IntegrateData’ functions with the default parameter. The top 10 PCs were used for clustering (resolution of 1.0) using the ‘FindNeighbors’ and ‘FindClusters’ functions and for visualization with UMAP. The classification of dorsoventral neuronal cell types of hdSpO was performed based on the combinatorial expression of known markers, as described previously38,39, by using ‘doCellPartition’ function (cell_level_min_step1 = 2, cell_level_min_step2 = 1; available at github.com/juliendelile/MouseSpinalCordAtlas).
For the organoid versus assembloid comparison, cells with more than 10,000 or less than 1,000 detected genes or cells with mitochondrial content higher than 15% were excluded. Genes that were not expressed in at least three cells in each library were not included. After normalization and variable gene detection, individual organoid samples (n = 4) and assembloid samples (n = 4) were integrated and clustered (30 PCs; resolution = 1).
For human DRG data, cells with more than 10,000 or less than 500 detected genes or cells with mitochondrial content higher than 10% were excluded. Genes that were not expressed in at least three cells in each library were not included. After normalization and variable gene detection, human DRG samples (n = 7) were integrated. UMAP visualization and clustering (30 PCs; resolution = 1) were performed. Human DRG samples (n = 7) and hSeO samples (n = 4) were also integrated. After removing putative stressed cells using Gruffi58, an additional round of integration, UMAP visualization and clustering (30 PCs; resolution = 2) were performed.
As a reference for label transfer from the human tissue datasets to four region-specific organoid datasets, the Braun et al.21 reference (available at https://github.com/linnarsson-lab/developing-human-brain) was downsampled, as described in our previous study59, and integrated with human DRG data using each donor as a batch. In the Braun et al. data, subregion labelling of hindbrain, pons and medulla was grouped into one as hindbrain. For label transfer of the dorsoventral subdomain information onto hdSpO, the Rayon et al. developing spinal cord data38 (GSE171892) were quality control (QC) filtered with the same criteria, as described in the original paper, and integrated using each donor as a batch. The prediction scores for each subregion were computed with the ‘FindTransferAnchors’ and ‘TransferData’ functions with 50 PCs. For each cell, the subregion with the highest prediction score was assigned as its predicted subregion. If the highest score was less than 0.5, the predicted subregion was marked as uncertain. Prediction proportions were calculated by dividing the number of cells in each organoid condition with a given subregion prediction by the total number of cells with that subregion prediction.
Generation of assembloids
To generate assembloids, regionalized organoids were differentiated separately and later assembled by positioning them in close proximity. For two-part assembloids, the organoids of interest were fused by placing them in 1.5-ml Eppendorf tubes for 2–3 days inside an incubator. For three- or four-part assembloids, the organoids of interest were integrated by placing them in tilted six-well ultra-low attachment plates (no. 3471; Corning) for 1 week in an incubator. After assembly, the assembloids were maintained in ultra-low attachment plates (24-well or six-well plates) with medium change every 4–7 days. For three- or four-part assembloids, the six-well ultra-low attachment plates were kept tilted in the incubator. The neural medium used for four-part assembloids was supplemented with BDNF (20 ng ml−1; 450-02; PeproTech), GDNF (25 ng ml−1; 450-10; PeproTech), NGF (25 ng ml; 450-01; PeproTech), l-ascorbic acid 2-phosphate trisodium salt (200 µM; 323-44822; Wako), IGF-1 (10 ng ml−1; 100-11; PeproTech) and cAMP (50 μM; D0627; MilliporeSigma). In the course of in vitro development, the timing of neuronal maturation differs along the rostro-caudal axis. This gradient has been taken into consideration especially when generating assembloids composed of organoids resembling spatially separated brain regions, such as hCO and hSpO8. Along the same line, we generally integrated more advanced hCO and hDiO (days 90–120) with earlier-stage hdSpO and hSeO (days 40–70).
Viral labelling and rabies tracing
Viral infection of organoids was performed, as described previously60. In brief, two or three organoids were placed in a 1.5-ml Eppendorf tube containing a 200-μl medium with 0.5 μl of the virus and incubated overnight at 37 °C and 5% CO2. The next day, 800 µl of fresh culture medium was added. The following day, neural organoids were transferred into the fresh culture medium in ultra-low attachment plates. For rabies virus retrograde tracing, organoids representing the ‘presynaptic’ part were labelled with AAV-DIO-tdTomato, and organoids representing the ‘postsynaptic’ part were separately labelled with Rabies-ΔG-Cre-GFP and AAV-EF1α::CVS-G. Two days after viral infection, the organoids were assembled. After 3 weeks of integration, assembloids were fixed with 4% paraformaldehyde and processed for immunocytochemistry. For rabies virus tracing and calcium imaging experiments, hdSpO were labelled with AAV-EF1α::fDIO-GCaMP6f, and hDiO were separately labelled with both Rabies-ΔG-Flpo-dsRedExpress and AAV-EF1α::CVS-G. For GCaMP virus infection of four-part assembloids, one single assembloid was cultured overnight in 200-μl medium containing 0.5 μl of the virus of interest, at 37 °C and 5% CO2. The next day, 4 ml of the fresh culture medium was added.
The viruses used in this study were AAV-DJ-hSYN1::EYFP (GVVC-AAV-16; Stanford University Neuroscience Gene Vector and Virus Core), AAV-DJ-EF1α::CVS-G (produced by Stanford University Neuroscience Gene Vector and Virus Core using Addgene plasmid no. 67528), AAV-DJ-EF1-DIO-tdTomato (GVVC-AAV-169; Stanford University Neuroscience Gene Vector and Virus Core), AAV-DJ-hSYN1::mTurquoise2 (produced by Stanford University Neuroscience Gene Vector and Virus Core using Addgene plasmid no. 99125), AAV-DJ-hSYN1::mCherry (GVVC-AAV-17; Stanford University Neuroscience Gene Vector and Virus Core), Rabies-ΔG-Cre-GFP (Salk), Rabies-ΔG-Flpo-dsRedExpress (Salk using Addgene plasmid no. 32650), AAV1-EF1α::fDIO-GCaMP6f (no. 128315-AAV1; Addgene), Lenti-hSYN1::jGCaMP8s61 (produced by VectorBuilder), AAV1-hSYN1::Cre (no. 105553; Addgene) and AAV-DJ-EF1α::DIO-GCaMP6s (GVVC-AAV-91; Stanford University Neuroscience Gene Vector and Virus Core).
Live calcium imaging from organoids or assembloids
To achieve single-cell-resolution live calcium imaging in intact 3D organoids or assembloids without significant sample movement during chemical injection, we acutely attached samples to ethyleneimine polymer (PEI)-coated coverslips before imaging. Autoclaved 12-mm coverslips were coated with 0.01875% PEI (no. 03880; Sigma) in water for at least 1 h at 37 °C and then washed three times with water before use. An organoid or assembloid was carefully positioned on top of a PEI-coated coverslip, and an L-shaped tubing-type Chamlide CMB chamber (CM-B12-1PB; Live Cell Instrument) was assembled. This chamber was connected to a peristaltic pump (no. 702027; Harvard Apparatus) to establish continuous suction through the outlet hole. To prevent sample drying, a vehicle solution was applied immediately after assembly. Organoids and assembloids with detectable expression of genetically encoded calcium indicators and displaying baseline activity were imaged.
The live imaging chamber was transferred to the confocal microscope stage (Leica STELLARIS 5 or SP8), and the samples were imaged using LAS X software (Leica) under controlled environmental conditions (37 °C and 5% CO2) using a ×5 or ×10 objective. We waited for 5–10 min and then started time-lapse live imaging at approximately 0.436 s per frame, with adjustments for each sample. For imaging spontaneous activity patterns, calcium activity was recorded for 3–5 min. To capture calcium responses after chemical injection, we recorded 60-s baseline activity. Following this, αβ-MeATP (no. 3209; Tocris) or capsaicin (no. 0462; Tocris) dissolved in vehicle was injected over 30 s at a slow speed (approximately 2 ml min−1) to minimize any potential movement of the samples.
For analysis, regions of interest (ROI) were manually drawn, and raw fluorescent intensities were exported using Fiji (v.2.14.0). Using MATLAB (v.R2023a), raw fluorescent intensities were transformed into relative changes in fluorescence: ΔF/Fbase = (F(t) − Fbase)/Fbase, where Fbase is the lower fifth percentile value of the session. The mean amplitudes of ΔF/Fbase from each cell for 60 s were compared before and after chemical injection.
As shown in Fig. 2g,h, a neural medium supplemented with BDNF (20 ng ml−1), GDNF (25 ng ml−1) and NGF (25 ng ml−1) was used as the vehicle solution. After 1 min of baseline imaging, 30 μM of αβ-MeATP or 3 μM of capsaicin was injected into the chamber.
As shown in Fig. 2k,l, a neural medium supplemented with BDNF (20 ng ml−1), GDNF (25 ng ml−1) and NGF (25 ng ml−1) was used as the vehicle solution. The hSeO were incubated with various concentrations of TNP–ATP (10, 100 or 100,000 nM) dissolved in the vehicle solution. After 1 min of baseline imaging, 30 μM of αβ-MeATP was injected into the chamber.
As shown in Fig. 3q,r, assembloids were acutely attached to a PEI-coated 12-well cell culture plate before the imaging. The plate was moved onto the microscope stage, and the imaging was done as the lid of the plate was open. A neural medium supplemented with BDNF, GDNF and NGF was used as the vehicle solution. After 1 min of baseline imaging in 1 ml of the vehicle solution, 500 μl of αβ-MeATP (90 μM) was added during imaging to make 30 μΜ of αβ-MeATP. After 6 min of imaging, the samples were incubated with NBQX (50 μM; no. 0373; Tocris) and APV (50 μM; no. 0106; Tocris) for 30 min in the incubator. Calcium response by αβ-MeATP in the presence of NBQX and APV was performed, as described above.
As shown in Fig. 4e,f, Dulbecco’s phosphate-buffered saline (DPBS) with calcium, magnesium (SH30264.01; Cytiva) or artificial cerebrospinal fluid (aCSF; containing 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.2 mM MgSO4, 1.5 mM CaCl2, 26 mM NaHCO3 and 10 mM d-(+)-glucose) with the addition of GlutaMAX (35050061; Gibco) was used as the vehicle solution. After 1 min of baseline imaging, 3 μM of αβ-MeATP was injected into the chamber.
As shown in Figs. 4k,l and 5f,g, aCSF was used as the vehicle solution.
The correlation between calcium traces was quantified using SCA42. Traces were first averaged over 1-s intervals and partitioned into 20-s-long segments. Pearson correlation coefficient (r) was computed between corresponding segments and averaged across all segments. This analysis was repeated for multiple time lags within the range of [−20, 20] s for each pair of calcium traces, with 1-s shift step. The peak correlation value across time lags was taken as a measure of synchrony between the calcium signals. This approach yields an accurate correlation estimate between signals on a short timescale, irrespective of common activations over longer periods of time.
Glutamate uncaging in assembloids
Αssembloids were imaged under environmentally controlled conditions (37 °C) using a ×5 objective in a confocal microscope (Leica SP8). For glutamate uncaging experiments, MNI-caged-l-glutamate (1490; Tocris) was used at a final concentration of 2.5 mM in aCSF. The FRAP software module of the Leica SP8 confocal microscope was used to uncage glutamate using ultraviolet light (405 nm). At a frame rate of 2.3 frames per second, one trial consisted of 50 frames for pre-stimulation, three frames of ultraviolet stimulation (in specified ROI) and 100 frames for post-stimulation; three trials were applied for one sample. Calcium responses were calculated by comparing the mean of ΔF/Fbase during 50 frames (21.8 s) before and after ultraviolet illumination, as described above, and averaged values of three trials were used for each sample.
Western blotting
Three to five organoids were collected in the same tube and considered as one sample. Whole-cell protein lysates for samples were prepared using the RIPA buffer system (sc-24948; Santa Cruz Biotechnology). Protein concentration was quantified using the bicinchoninic acid assay (Pierce; catalogue no. 23225; Thermo Fisher Scientific). For electrophoresis, 30 µg of protein per lane was loaded and run on a 4–12% Bis-Tris PAGE gel (Bolt 4–12% Bis-Tris Protein Gel; catalogue no. NW04122BOX; Invitrogen) and transferred onto a polyvinyl difluoride membrane (Immobilon-FL; EMD Millipore). Membranes were blocked with 5% BSA in tris-buffered saline with Tween (TBST) for 1 h at room temperature and incubated with primary antibodies: anti-glyceraldehyde 3-phosphate dehydrogenase (mouse; 1:5,000; GTX627408; GeneTex) for 1 day and anti-NaV1.7 (rabbit; 1:1,000, ASC-008; Alomone Labs) for 6 days. Membranes were washed three times with TBST and then incubated with near-infrared fluorophore-conjugated species-specific secondary antibodies (Goat Anti-Mouse IgG Polyclonal Antibody (IRDye 680RD; 1:10,000; 926-68070; LI-COR Biosciences) or Goat Anti-Rabbit IgG Polyclonal Antibody (IRDye 800CW; 1:10,000; 926-32211; LI-COR Biosciences)) for 1 h at room temperature. Following secondary antibody application, membranes were washed three times with TBST and once with TBS and then imaged using a LI-COR Odyssey CLx imaging system with Image Studio Software (v.5.2; LI-COR). Western blots were analysed using the Image Studio Software or Fiji (v.2.14.0) (see Source data for full blots).
Axon projection imaging and analysis
EYFP+ projections in target regions were imaged under environmentally controlled conditions in intact assembloids using a Leica TCS SP8 or Leica STELLARIS 5 confocal microscope with a motorized stage. Assembloids were transferred to a well in a 24-well glass bottom plate (P24-0-N; Cellvis) in a cell culture medium and incubated in an environmentally controlled chamber for 15–30 min before imaging. Images were taken using a ×10 objective to capture the entire target area at a depth of 100 μm. Projections were quantified using Fiji (v.2.14.0). ROI were manually drawn to cover the target area to be measured in max projection confocal stacks. The percentage of fluorescence-positive pixels over the target region area was calculated in binary images.
Mouse primary tissue dissection and imaging
Mouse tissue samples were obtained using protocols approved by Stanford University’s Administrative Panel on Laboratory Animal Care. Timed pregnant CD1 mice with 11 days of gestation were obtained from Charles River Laboratories and maintained on a 12-h light–dark cycle, an ambient temperature of 20–26 °C and a humidity of 30–70%. We anaesthetized pregnant mice with isoflurane on day 13 of gestation, and E13.5 embryos were used in this study without knowing their sex. DRG were dissected from E13.5 embryos, placed en bloc onto PEI-coated 12-well plates and cultured in DRG culture medium (neural medium supplemented with BDNF, GDNF, NGF and 10% FBS). Calcium imaging was performed the next day after dissection.
As shown in Extended Data Fig. 3a–d, mouse DRG were incubated with Calbryte 520 AM (10 µM; no. 20653; AAT Bioquest). After 45 min, the medium was changed to 1 ml of the DRG culture medium. To image the calcium activity of mouse DRG, a whole-cell culture plate containing mouse DRG was moved to an environment-controlled confocal microscope stage. Imaging was performed when the lid of the plate was opened. After 1 min of baseline line imaging, 500 μl of αβ-MeATP (90 μM) or capsaicin (10 μM) dissolved in the DRG culture medium was added into the well by pipetting without touching the cell culture plate (to prevent XYZ movement). Imaging was performed for 6 min. The final concentrations of the agonists were 30 μM (αβ-MeATP) and 3.3 μM (capsaicin).
As shown in Fig. 2l,m, mouse DRG were incubated with Calbryte 520 AM, as described above, and additionally incubated with various concentrations of TNP–ATP (10, 100 or 100,000 nM; no. 2464; Tocris) dissolved into DRG culture medium for 30 min in the incubator. After 1 min of baseline imaging with TNP–ATP, 500 μl of αβ-MeATP (90 μM) in the DRG culture medium was added into the well. Imaging was performed for 6 min.
Extracellular recordings
Extracellular recordings were performed, as described previously11. Assembloids were embedded into 3% low-melting gel agarose (IB70056; IBI Scientific). Embedded samples were placed on a Brain Slice Chamber-2 (S-BSC2; Scientific Systems Design) and perfused with aCSF (bubbled with 95% O2 and 5% CO2) at 37 °C. The temperature was controlled and retained at 37 °C by connecting to a Proportional Temperature Controller PTC03 (S-PTC03; AutoMate Scientific). Acute 32-channel P-1 probes with two shanks (ASSY-37 P-1; Cambridge NeuroTech) were connected to an Acute probe adaptor; 32-channel Samtec to Omnetics (ADPT A32-Om32; Cambridge NeuroTech). Baseline activities were acquired using the Intan 1024ch recording controller (Intan Technologies) at 30,000 Hz for 5 min using RHX software (v.3.1.0). Raw recording data were processed using Intan Technologies code and analysed using MATLAB (v.R2022a; MathWorks).
To analyse extracellular recording data, band-pass filtering (350–2,000 Hz) was performed followed by common median referencing. A threshold (six times multiplied by s.d.) for each channel was used to count neuronal activities. Activity from hdSpO, hDiO or hCO was counted ± 500 ms of each activity of hSeO channels. Activity counts around the peak area (P) − activity counts in the base area (B)/(P + B) were used as co-activation index44. Parameters (bin size, 5 ms; plot size, ±500 ms) were used to draw the representative cross-correlograms.
Genome editing of hiPS cells to generate SCN9A KO and gain-of-function hiPS cell lines
For generating SCN9A KO cells, three single guide RNAs (sgRNAs) were designed and synthesized by Synthego to induce one or more fragment deletions. The sgRNA sequences are as follows: 5′-AGCUCGUGUAGCCAUAAUCA-3′, 5′-CGUGUGUAGUCAGUGUCCAG-3′ and 5′-UUCUCUUGGUACUCACCUGU-3′. The hiPS cells were dissociated with Accutase, and 0.5 million cells were mixed with 300 pmol sgRNAs and 40 pmol Cas9 protein (SpCas9 2NLS Nuclease (1,000 pmol); Synthego). Nucleofection was performed using the P3 Primary Cell 4D-Nucleofector X Kit S (V4XP-3032; Lonza), a 4D-Nucleofector Core Unit and the X unit (program CA-137; Lonza). Cells were then seeded onto vitronectin-coated six-well plates in Essential 8 Medium supplemented with the ROCK inhibitor Y27632 (10 µM). Essential 8 Medium was used for daily medium change. The genotype of pooled cells was determined by PCR with the primer set Fw, 5′-CGAGAACTACCCATATTATTAGTGATGG-3′; Rv, 5′-CCAAGAACTATCACAAAACGTCTGT-3′, and Sanger sequencing (GENEWIZ) was performed with the reverse primer.
SCN9A gain-of-function cells were generated by Bayspair using two-step scarless genome editing62. Genotyping was performed using PCR with the primer set Fw, 5′-GTGTTTGGAGACCCATGTTTC-3′; Rv, 5′-GAAGTAGTAGTGTCTGAGGGAGATC-3′, and Sanger sequencing (GENEWIZ) was performed with the reverse primer.
Patch-clamp recordings
Patch-clamp recordings were conducted, as described previously11. In brief, hSeO were first infected with Lenti-hSYN1::ChR2-EYFP before assembly and then integrated with hdSpO to create hSeO–hdSpO assembloids. These assembloids were subsequently infected with AAV-DJ-hSYN1::mCherry and placed onto cell culture inserts (0.4-µm pore size; 353090; Corning) positioned in six-well plates to form flattened assembloids. Recordings were typically performed on days 148–164 (daf 99–110). mCherry-positive neurons within the hdSpO were identified using an upright microscope. For optogenetic stimulation, whole-field light-emitting diode illumination (460 nm; 5–20 ms duration; maximal power; CoolLED) was applied through a ×40 objective, and oEPSCs were recorded at a holding potential of −70 mV.
Patch-clamp recordings in whole-cell configuration were performed at room temperature on spinal organoid neurons, which were visualized using an upright microscope (Scientifica) equipped with an INFINITY2 charge-coupled device camera and INFINITY Capture Software (Teledyne Lumenera). The external aCSF recording solution contained 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3 and 10 mM d-(+)-glucose. During recordings, the assembloids were perfused with the aCSF recording solution (bubbled with 95% O2 and 5% CO2). Thick-walled borosilicate pipettes (resistance of 6–9 MΩ) were filled with an internal solution consisting of 135 mM K-gluconate, 20 mM KCl, 0.1 mM EGTA, 2 mM MgCl2, 2 mM sodium-ATP, 10 mM HEPES and 0.3 mM sodium-GTP (pH adjusted to 7.28 with KOH; approximately 302 mOsm). Data were acquired using a MultiClamp 700B Amplifier (Clampex 10.7; Molecular Devices) and a Digidata 1550B Digitizer (Molecular Devices), low-pass filtered at 2 kHz, digitized at 20 kHz and analysed with Clampfit (v.10.7; Molecular Devices).
Statistics and reproducibility
Data are presented as mean ± s.e.m. unless described otherwise. Raw data were tested for normality of distribution, and statistical analyses were performed by two-tailed unpaired t-test, two-tailed Mann–Whitney test, two-tailed paired t-test, two-tailed Wilcoxon matched-pair signed-rank test, Wilcoxon signed-rank test, one-way ANOVA and Kruskal–Wallis test with multiple comparison tests depending on the dataset. Sample sizes were estimated empirically. GraphPad Prism v.10.0.0 or MATLAB (v.R2023a; MathWorks) was used for statistical analyses. Data shown in representative experiments were repeated with similar results in multiple independent experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.