In the 30 years since exoplanets were first reported1, telescopes have revealed thousands of them. One of the most surprising things about these objects, which orbit stars other than our Sun, has been their diversity: they range from scorching lava planets with atmospheres of vaporized rock to ocean worlds, and some might even look like Earth.
The James Webb Space Telescope (JWST), which was launched in 2021, has been revolutionizing our understanding of the atmospheres of these planets, showing details of clouds and haze that were previously unfathomable2. Scientists are starting to learn how winds and turbulence transport compounds on worlds beyond the Solar System3, and are seeing chemical processes that are unlike those found on Earth4.
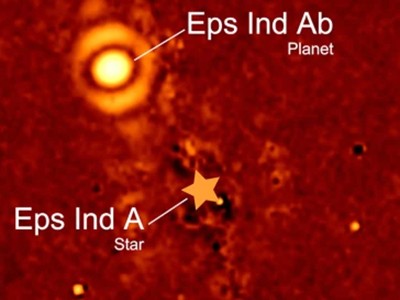
This glowing speck is a freezing exoplanet six times the size of Jupiter
Such insights promise to tell us more about how planets form — and perhaps the origins of life. But many of the findings, such as hints of unfamiliar compounds, are proving challenging to understand.
The space-science community needs help from scientists in other fields to find answers. Here, we call for closer collaborations between exoplanet researchers and those who work on Earth’s and other planetary atmospheres, as well as stronger links between experimentalists, modellers and astronomers.
Focus on photochemistry
To understand the molecules and processes at play in exoplanet atmospheres, it is crucial to consider photochemistry — chemical reactions driven by high-energy light from nearby stars. One of JWST’s first observations was of WASP-39 b, a hot and puffy gas giant the size of Jupiter, orbiting a Sun-like star some 215 parsecs (700 light years) away. The electromagnetic spectrum of its atmosphere revealed clear signatures of molecules that astronomers expected to find on such a planet — including carbon dioxide and water. But an unfamiliar opaque region at a wavelength near 4 micrometres puzzled them.
Because the data set was open source, hundreds of astronomers came together in 2022 in a frenzied collaboration, hosted on the messaging platform Slack, to identify the chemical species responsible for that opaque region. Scores of possibilities were tested before it became clear that it was sulfur dioxide. This was surprising, in that this compound should be rare in hydrogen-dominated gas giants5. Only one process was found to generate sulfur dioxide in high enough concentrations: photochemistry.
Similar mysteries will present themselves in the future. Modellers, working closely with theoretical and experimental chemists, must improve the photochemical models used to interpret these data accurately. Crucially, there is a lack of comprehensive data on how reaction rates and absorption cross-sections change with temperature and pressure for these exotic atmospheres, as well as a lack of data for molecules that are unfamiliar to our Earth-centric experience.

The 18-segment gold mirror of the James Webb Space Telescope. Credit: Desiree Stover/NASA
Make models go further
Detecting a particular molecule in an atmosphere is insufficient to deduce how it was formed. It could have been transported up from deep in the planet’s interior, or many compounds and chemical pathways could have led to its presence, possibly through reaction pathways different from those of the Solar System. But current models are anchored in the physics and chemistry of processes happening on planets local to us, from the gas giants to rocky terrestrials. The models might therefore be misrepresenting, or entirely missing, some chemical or physical processes at play in exoplanet atmospheres.
Physical factors that need to be better represented in models include the transport and exchange of molecules between a planet’s atmosphere and its surface or interior. The way in which a planet rotates — about its axis and around its host star — can affect its atmospheric chemistry. Some exoplanets, for example, orbit their host star in such a way that only one side is ever illuminated, resulting in a permanent, hot dayside and a permanent, cold nightside. This gives a very different distribution of gases to what is found on Earth. Better numerical models will improve the global framework that describes the physical movement and observable composition of these atmospheres.
JWST’s best images: spectacular stars and spiralling galaxies
Another cornerstone of models are photochemical parameters, and these also need a careful look. They represent how molecules respond to incoming stellar radiation and how they react with each other — through which reactions, and how fast. To make things more complicated, these parameters can change with pressure and temperature in the atmosphere. The atmospheric environments of currently observed exoplanets diverge from those of planets in the Solar System, and therefore models must simulate a vast range of temperatures, pressures and chemical conditions. This means measuring or calculating parameters that will represent more accurately what happens in exoplanet atmospheres. Reaction parameters are ideally calculated from reaction rates that have been measured in laboratory experiments under conditions relevant to the planet of interest, or from theoretical simulations based on quantum mechanical first principles. However, there are known reactions that lack parameters (and therefore models often use best guesses), and probably a slew of reactions that have yet to be discovered.
Here, we propose two pathways — experimental and theoretical — to expand the catalogue of reactions and their parameters specifically for exoplanets.
Expand interdisciplinary research
Most lab-based research into exoplanet atmospheres has focused on the light-absorption signatures of specific gases such as titanium oxide and phosphine6, or on haze particles, suspended in gas, that are produced from a variety of compounds and can affect the propagation of stellar radiation. Current experiments are conducted in chambers that can replicate the range of observed exoplanet atmospheric temperatures, pressures and chemical compositions, using lamps or plasma to simulate stellar radiation, stellar flares or lightning7.
These studies show the diversity and complexity of chemical products that can be generated from very simple exoplanet analogue atmospheres, and they provide optical parameters to help interpret observations. But photochemical models are used to predict the chemical make-up of atmospheres by leveraging entire reaction networks — sets of hundreds to thousands of individual reactions that connect different molecules to each other. The current lab studies have not provided the quantitative information needed to develop reaction networks for exoplanets.
To build up this capability, the exoplanet community must first identify the lab technologies required for the measurements they need, and there has already been significant progress8,9. To study reaction parameters, for example, scientists must ideally examine specific molecules as they react, and the products that they form.
Equipment from labs that specialize in Earth-based atmospheric chemistry and combustion can be repurposed to some extent. Using combustion-science reactors and shock tubes, together with spectroscopic or mass-spectrometric detection techniques, would immediately widen the ranges of pressures and temperatures that can be studied. And rather than oxygen, which is abundant in Earth’s atmosphere, these techniques can be tuned to study species such as sulfur compounds and methane behave, which might be more abundant elsewhere. Similarly, expanding the user community of Earth-science labs to include exoplanet scientists would help to maximize the scientific return on this expensive infrastructure. In the longer term, however, exoplanet research needs dedicated facilities.
JWST reveals first evidence of an exoplanet’s surprising chemistry
New techniques will also be required to measure exotic target molecules or particles specifically for exoplanets. The science will be shaped by, for example, the proposed Habitable Worlds Observatory, which will focus on ultraviolet and near-infrared radiation, or the mid-infrared Large Interferometer for Exoplanets. These complementary space-based telescope concepts will help to provide the improved observational data needed to test and constrain the chemical and physical models of exoplanet atmospheres. This has been the case in other fields, for example, with the ion-imaging technique created at Sandia National Laboratories’ Combustion Research Facility in Livermore, California10. The technique records the velocity of charged particles that result from a molecule being broken apart by light or in a collision, and was originally developed to advance our knowledge of combustion. Future observations could motivate similar technological developments that will provide precise, molecular-level parameters to inform reaction networks used in the photochemical models of exoplanet science. What’s more, the development of technologies for exoplanet research would ultimately benefit the Earth-science and combustion-science communities, too.
Use first principles
There are, however, reactions and molecules that are too difficult or dangerous to probe experimentally, even in lab settings. The extreme high and low pressures of exoplanet atmospheres, or reactions that are very slow, can be hard to study owing to the limitations of the instruments used. For slow reactions, minute changes in molecular concentration can be smaller than the random noise in the instrumentation; at low pressures, molecular densities might be below the detection limit.
In such cases, chemists can apply theoretical tools to calculate key parameters over a wide range of temperatures and pressures. With increasing computer power and the integration of artificial intelligence, such calculations are rapidly improving in terms of accuracy and efficiency11. Yet, reactions that involve large molecules or heavy atoms remain hard to predict accurately.