This February, 14 lorries set out from Wilmington, North Carolina, with a toxic cargo: more than 150 tonnes of grit-like carbon that had soaked up harmful chemicals from the city’s drinking water.
The lorries took the carbon to one of the closest available ‘reactivation’ kilns, 1,200 kilometres north in Buffalo, New York. There, temperatures of nearly 1,000 °C burnt off the chemicals, breaking them into simple gas molecules, which were later turned into minerals. This month, the refreshed carbon will ride the lorries back down south.
The manager of Wilmington’s drinking-water plant, Benjamin Kearns, says he checks the weather forecast for Buffalo all winter — fearing disruptions to his water-purifying operations, which depend on a carefully timed supply of fresh carbon every month. “If there’s a snowstorm, I am concerned,” he says.
The carbon, technically called granular activated carbon (GAC), is at the heart of a US$43-million system that began operating in 2022 to rid Wilmington’s drinking water of PFASs, or per- and poly-fluoroalkyl substances. These synthetic chemicals now pervade the world — they’re used to make computer chips, lithium-ion batteries, medical devices, stain-resistant textiles and smudge-proof coatings, among many other products — and some of them endanger human health. Also known as forever chemicals, they resist natural destruction because of their strong carbon–fluorine bonds.
Kearns’s plant is in the vanguard of an almighty remediation effort. In April 2024, the US Environmental Protection Agency (EPA) put strict nationwide limits on the concentrations of six PFASs in drinking water. The agency estimates that the rule will reduce PFAS exposure for around 100 million US residents.
How best to get rid of PFASs is now a multibillion-dollar question. The EPA estimated that US utilities might have to spend up to $1.5 billion annually for treatment systems; an industry group that is suing the agency argues that costs could be up to $48 billion over the next 5 years. Utilities must have systems in place by 2029.
European nations have rules restricting PFAS levels in drinking water, too. European Union rules take effect from 2026, but allow higher concentrations than the EPA does; however, countries such as Denmark and Germany have set stricter limits.
The concern and the expectation of a booming market for cleaning up PFAS pollution has sparked a rush for better ways to capture and destroy forever chemicals. Although GAC does work — it is a porous material (or sorbent) that can trap and house pollutants — it doesn’t capture all PFASs equally well. Trucking carbon around so that the collected PFASs can be destroyed in reactivation kilns also exacerbates climate change, adds Frank Leibfarth, a polymer chemist at the University of North Carolina at Chapel Hill.

Drinking water is filtered through nearly 4 metres of granular activated carbon in huge tanks at the Sweeney Water Treatment Plant.Credit: Cape Fear Public Utility Authority
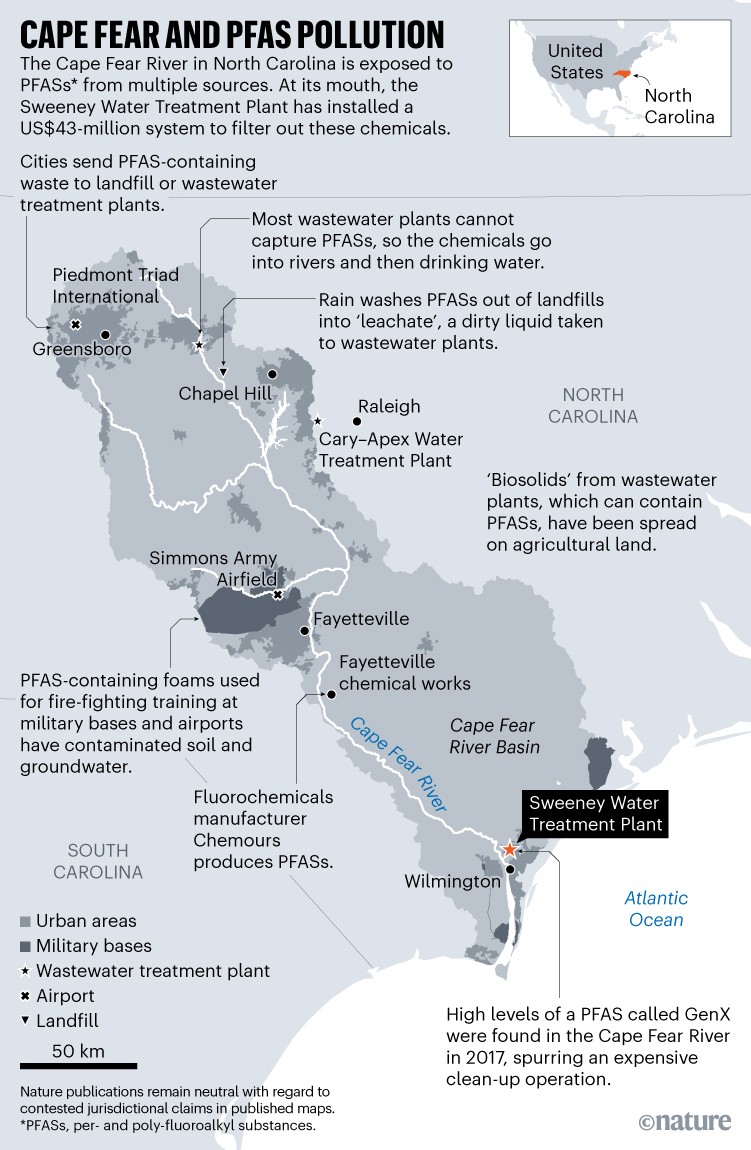
And although the EPA has focused on drinking water, scientists want to stop PFASs from ever reaching the water by removing them from other environmental sources. The industrial facilities that produce and use PFASs, ranging from fluorochemical manufacturers to paper and textile mills, often send their waste to municipal wastewater (sewage treatment) plants. But these aren’t usually equipped to remove PFASs, so their outflow adds forever chemicals into rivers. From there, the PFASs can reach drinking water directly or do so indirectly by infiltrating soils.
The sludge that is left over from sewage treatment also accumulates PFASs. In some parts of the world, this nutrient-rich sludge, known as biosolids, has been spread onto farmland as fertilizer. In states such as Maine, farms that yield PFAS-tainted food have shut down. And a type of fire-fighting foam that is formulated with PFASs has contaminated soils and seeped into groundwater around military bases and airports worldwide, because of its frequent past use in fire-training exercises there.
With looming deadlines, academic researchers and companies are developing methods to gather and destroy PFASs from these sources. “Loads of evolving techniques are out there,” says PFAS specialist Ian Ross at CDM Smith, an engineering firm in Boston, Massachusetts, that works on PFAS remediation.
Capturing contaminants
On the second floor of Kearns’s facility — the Sweeney Water Treatment Plant — drinking water held in up to eight concrete tanks sinks silently through nearly four metres of GAC. “It’s amazing how much carbon you need to treat for PFAS,” says Orlando Coronell, an engineer at the University of North Carolina at Chapel Hill who is collaborating with Leibfarth to test a new sorbent at the facility.
Operated by the Cape Fear Public Utility Authority (CFPUA), this plant serves 200,000 people in the coastal city of Wilmington. It draws water from the Cape Fear River, which in 2017 was shown to have high levels of one of the six EPA-regulated PFASs, called GenX (see ‘Cape Fear and PFAS pollution’). The molecules had come from 160 kilometres upstream, where fluorochemical manufacturer Chemours makes PFASs for electronics and battery manufacturing, among other uses, and had discharged them into the river.
Source: RTI International (Adapted from https://go.nature.com/429E3DL)
After the CFPUA installed the sorbent system, levels of GenX and several other PFASs fell (and are below the new EPA limits). While the system was being designed and constructed, North Carolina’s state environmental agency sued Chemours, and a local non-profit group added pressure by suing both organizations together. The parties agreed to settle: Chemours denied wrongdoing, but installed better controls on its PFAS emissions, including a 1.6-kilometre-long underground barrier wall paired with a GAC filtering system that collects surface and groundwater near the plant and removes PFASs. The firm says it has invested more than $400 million at its plant to remediate PFAS emissions and limit future ones. The CFPUA is currently suing Chemours to pay for the Sweeney filtration system.
GAC is generally effective, but it is a ‘broad-spectrum’ sorbent that traps everything it attracts into its hydrophobic (water-repellent) pores, not just PFASs, says Coronell. The Sweeney plant receives water with much higher levels of dissolved organic matter than of PFASs, which compete for space in GAC’s pores. The six molecules on the EPA’s list stick well enough, but any PFAS with a shorter, hydrophobic fluorine-bearing tail does not. As GAC’s pores fill up, short-chain PFASs can break through the pores and re-enter drinking water.
In particular, ultrashort-chain PFASs (those with a three-carbon fluorinated tail or shorter) are worrying researchers(see ‘PFAS pollutants’), because the molecules are being found in waters downstream of Chemours and near semiconductor manufacturing facilities1. After the CFPUA detected two ultrashort PFASs in its drinking water after treatment, it began switching out the GAC about every 200 days, instead of the roughly 300 days it had used when capturing GenX. That has almost doubled its carbon-regeneration costs.
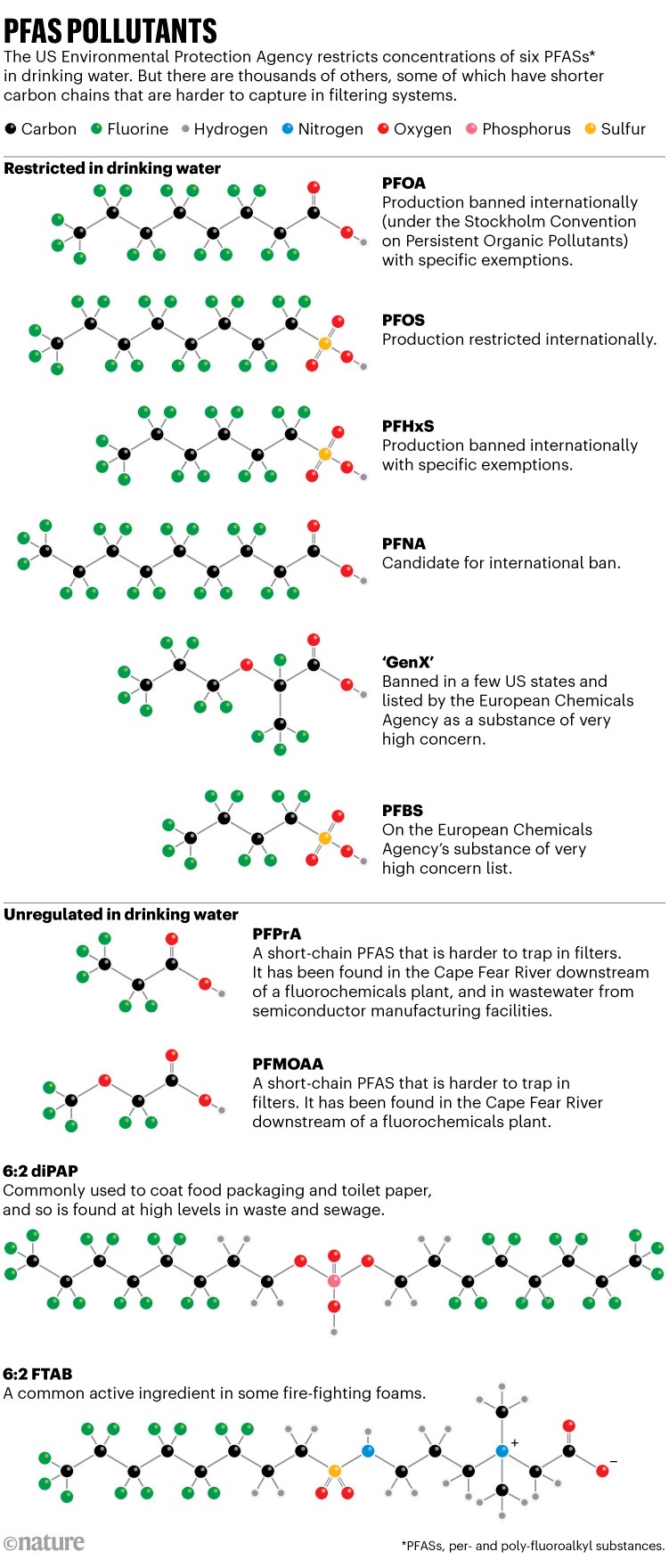
Other established ways to capture PFASs have pros and cons. A type of sorbent called ion-exchange resin traps contaminants broadly through electrostatic interactions: the six EPA-regulated PFASs all carry a negative charge, and they stick by trading places with a negatively charged component on the resin.
Less resin is needed to treat the same amount of water than with GAC, but it costs five to six times more, says Detlef Knappe, an environmental scientist at North Carolina State University in Raleigh. Nitrate salt ions in the water can clog the resin and reduce its cost-effectiveness, and the resins are used just once in drinking-water facilities, because cleansing them often involves washing in methanol, an unacceptably toxic solvent.
Another method uses membranes to separate contaminants from water. In reverse osmosis, mechanical pressure forces water through a membrane with tiny pores: water that is almost pure passes through, while everything else stays on the other side in a gradually saltier mix. Membrane systems are more expensive to build — reverse osmosis was three times the cost of a sorbent system when the CFPUA evaluated the options. (But if sorbents had to be changed more frequently, then membrane systems would become cost-effective, says Knappe.) Reverse osmosis also generates massive volumes of a watery, PFAS-laced brine that is difficult to manage.
Targeted PFAS traps
Many researchers are inventing sorbents that can trap PFASs more selectively, often involving multiple chemical interactions at once. On the first floor at the Sweeney plant, beneath the GAC tanks, Coronell and Leibfarth are testing a proprietary sorbent. So far, it has lasted three times as long as the CFPUA’s GAC and 40% longer than a top-performing ion-exchange resin before the short-chain molecules have broken through. One possibility, says Kearns, is to add a layer of a new sorbent to capture escapees from GAC, thereby lengthening the time between trips to the reactivation kiln.
Some researchers are testing their sorbents on dirtier, more complex PFAS sources, such as wastewater. The dirtiest of all is the liquid that pools at the bottom of a landfill (landfill leachate), which must be pumped out and treated, often by being transported to the nearest wastewater treatment plant by lorry. “It’s pretty gross,” says William Dichtel, a chemist at Northwestern University in Evanston, Illinois, who plans to test a sorbent on the leachate.
In general, sorbents capture long-chained PFASs better than short-chained ones. Costly membrane systems might prove necessary for waters enriched in short-chained PFASs: one study2 found that nanofiltration, which uses membranes with slightly larger pores and produces less waste than reverse osmosis, captured more than 90% of ultrashort-chain PFASs from semiconductor wastewater.
Another idea is to reconfigure GAC itself. The material’s pores are irregularly shaped, but carbon chemist Pan Ni at the University of Missouri in Columbia and his colleagues have reported preliminary work at a conference suggesting that the pores could be aligned into nano-sized channels instead. With the right channel diameters, GAC might begin targeting just the short-chained molecules.
Destroying captured PFASs
Every sorbent eventually becomes full. How best to destroy the accumulated PFASs is now a key question and a billion-dollar market.
Utilities that choose to clean their water using GAC could follow the Sweeney example and drive it to a reactivation kiln. An alternative is incineration, which is also a common way to dispose of spent single-use resins. Incineration simply destroys materials by burning them in the presence of oxygen — “a runaway reaction”, says Knappe — whereas GAC reactivation is controlled and works without oxygen.
Ideally, both kinds of treatment would break every carbon–fluorine bond and release fluorine as hydrogen fluoride gas. The gas could then pass through ‘scrubbers’ containing alkaline reagents similar to baking soda to convert it into harmless minerals such as sodium fluoride.
But it is not clear that the PFASs are completely mineralized, because some laboratory studies can’t match up the mass of the fluorine going in with that recovered from the products3. This suggests that some PFASs might have been broken up only into smaller gaseous PFAS molecules, which are harder to capture and might be spread into the air.
Such gases can, however, be captured with the types of filter already installed at incinerator facilities that routinely deal with hazardous waste, says AnnieLu DeWitt, an analytical chemist at Clean Harbors, a waste-management firm headquartered in Norwell, Massachusetts, that specializes in hazardous-waste incineration and landfill.
Tests in which PFASs were incinerated with added calcium minerals suggest that fluorine gets locked effectively into calcium fluoride. In Australia, some PFAS-containing wastes have been fed to cement kilns, which run at high temperatures and contain lots of calcium. However, total mineralization remains unproven.
Because of questions over the effectiveness of incineration, the US Department of Defense has temporarily forbidden its facilities from incinerating fire-fighting foam that has high concentrations of PFASs. The department is working with the EPA and Clean Harbors to check whether incineration produces some PFAS gases. If not, the expectation is that incineration will become the go-to technique, says Ross. In the meantime, a bevy of start-up firms, often spun out of academic labs, have developed alternative ways to destroy PFASs. Many of these use high-energy conditions to rip the molecules apart. The firms say that the techniques can treat fire-fighting foam and PFAS-laced brines or biosolids that aren’t suitable for incineration.
Vials of samples containing GenX, a PFAS chemical, in an EPA analysis lab in Cincinnati.Credit: Joshua A. Bickel/AP/Alamy
A Pennsylvania start-up firm called OnVector uses plasmas (ionized gases) to break the molecules apart, while the company 374Water in Morrisville, North Carolina, uses supercritical water (water that behaves like a gas and a liquid, owing to high pressure and temperature). A technique being commercialized by Aquagga, a firm in Washington state, uses water at a lower temperature and pressure than other firms do, but adds an alkali chemical to kick-start the PFAS destruction.


