As early as next month, a trial crop of hardy sorghum could start sprouting under a mesh containment canopy in eastern Australia. The plants might seem ordinary, but there’s something revolutionary hiding in their genes.
If the trial is a success, each plant will bypass sexual reproduction and will set seed to thousands of clones in each flower head.
The Hy-Gain sorghum trial is the culmination of decades of work for plant physiologist Anna Koltunow at the University of Queensland in Brisbane, Australia, who began research to make ‘sexless seeds’ in the early 1990s. The technology exploits a quirk of nature — known as apomixis. More than 300 species of flowering plant naturally produce clonal seeds through apomixis, but none are important grains. Researchers say that the quest to bring apomixis to plants such as sorghum, rice and maize (corn) is now on the brink of transforming agriculture.
How to climate-proof crops: scientists say the secret’s in the dirt
“It definitely will lead to a revolution,” says Kejian Wang, a geneticist at the China National Rice Research Institute in Hangzhou, who is working on separate apomixis experiments.
Koltunow says her work promises to give smallholder farmers in sub-Saharan Africa access to affordable high-yielding sorghum (Sorghum bicolor) and cowpea (Vigna unguiculata) crops. Those farmers could save the clonal seeds to sow for several years, further reducing costs. These are some of the reasons that Koltunow’s research is supported by the Gates Foundation, a charity in Seattle, Washington.
Another collaborator in the Hy-Gain project is the multinational seed company Corteva Agriscience, based in Indianapolis, Indiana. That’s because apomictic plants are a potential boon for seed companies selling to large-scale agriculture markets across the world. Apomixis could slash the time needed to create new varieties, and fix desirable traits in plants that produce their own clones. “The decrease in cost [could be] huge,” for those companies, says Koltunow.
Market-ready clonal seed production is within reach in a handful of species, including rice — a staple grain that feeds more than half of the world’s population. There has been a flurry of patent applications in the past few years for a variety of apomictic crop plants. Yet researchers say that some key hurdles remain before these advances can truly take root. “Now that we’ve gotten it to work as a concept, we need to fine tune things,” says Venkatesan Sundaresan, a plant reproductive biologist at the University of California, Davis.
Fixing vigour
The arrival of sexless seeds for growing crops could transform much of agriculture, foremost the production of hybrid seeds. For some of the main crops grown across the world, such as maize, rice and tomatoes, farmers have been sowing hybrid seed for generations.
When two parent varieties are crossed through sexual reproduction, the resulting hybrid offspring typically outperform both of their parents — a phenomenon known as hybrid vigour. Between 1930 and the mid-1990s, yields from maize crops in the United States increased sevenfold, partly because farmers adopted the practice of sowing hybrid seeds to produce row after row of uniformly robust plants.
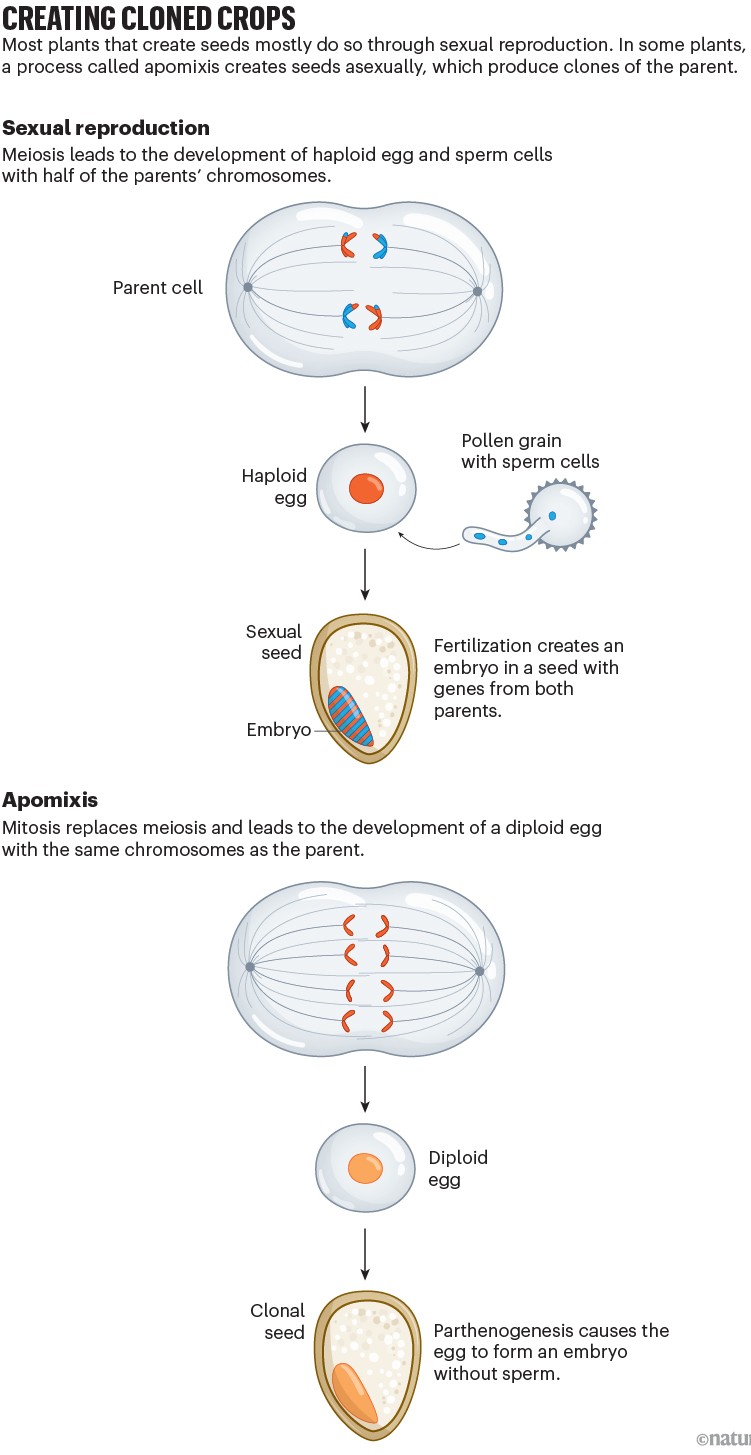
But making hybrids is laborious, time-consuming and expensive, and hybrid seeds must be created — and purchased — anew each year. That’s because if hybrids are left to self-pollinate, they produce a mish-mash crop of plants of varying quality, the result of sexual reproduction mixing and matching the plant’s genes into new combinations (see ‘Creating cloned crops’).
Source: Wang, Y. & Underwood, C. J. Curr. Biol. 33, R293–R295 (2023)
With apomixis, hybrid vigour would be ‘fixed’ in place, because the hybrids would clone themselves, providing breeders — and farmers — with an endless supply of high-quality, identical plants. This could also help in the production of crops such as wheat and soya bean, for which it has proven too difficult to produce hybrids. Apomixis would make it possible to develop elite varieties of these crops in which traits are fixed from one generation to the next.
By the 1940s, researchers had worked out that apomixis had a genetic basis. But it wasn’t until the 1990s that Koltunow and others recognized that the trait doesn’t override sexual reproduction. Rather, it is “a kind of altered sexual pathway”, says Ueli Grossniklaus, a plant geneticist at the University of Zürich, Switzerland. In that pathway, two innovations are necessary: the cell divisions during the formation of egg and sperm cells need to be disrupted; and an embryo needs to form independently of fertilization. Grossniklaus’s team conducted several experiments in which plants were deliberately mutated to see whether any mutants exhibited these changes.
It wasn’t until 2009 that researchers successfully disrupted the sexual cell-division process, known as meiosis, to mimic what occurs in natural apomicts1. Raphael Mercier, a plant geneticist then at the National Institute for Agricultural Research in Versaille, France, was trying to understand how mitosis — the simple process in which a cell divides into two identical cells — evolved into the more complex cellular dance of meiosis, a key part of sexual reproduction. Meiosis involves two cell divisions instead of one, and the resulting egg and sperm cells contain half the usual number of chromosomes, so that when combined during fertilization, the full complement of chromosomes is restored.
Using Arabidopsis thaliana, a small cabbage relative that has become the workhorse of plant genetics laboratories, Mercier identified a gene involved in one of the three crucial innovations that turned mitosis into meiosis during evolution. He then disrupted that gene, along with genes that are crucial for the other two pillars of meiosis, to see whether meiosis could be turned back into mitosis.
How farming could become the ultimate climate-change tool
“It worked just [as] expected,” says Mercier, now at the Max Planck Institute for Plant Breeding Research in Cologne, Germany. In flowers of the resulting triple mutant — dubbed MiMe, for mitosis instead of meiosis — sperm and egg cells contained the same set of chromosomes as their parent, having been made through the simpler cell division of mitosis rather than the more complex meiosis1. Mercier says that he immediately recognized the potential of MiMe for engineering apomixis in plants. He and his colleagues followed up with MiMe rice2 in 2016, and MiMe tomatoes3 in 2024. The plants could reproduce, but because the egg and sperm cells contained twice the normal number of chromosomes, the progeny also received a double dose of chromosomes, leading to decreasing fertility in each generation. It was a step in the right direction but only half of the apomixis puzzle.
Grossniklaus and his team discovered an alternative to MiMe in maize. A screen of 60,000 mutants turned up a single gene, called non-reductive in female4 (nrf4), that, when disrupted, causes about one-third of egg cells to be formed through mitosis instead of meiosis. This work, including the formation of the first clonal seed in a crop species using the nrf4 mutant, is covered by a patent4.
Virgin birth
The second essential component of apomixis is parthenogenesis, by which an embryo forms directly from an unfertilized egg cell — no male required. In 2006, Peggy Ozias-Akins, a molecular geneticist at the University of Georgia in Tifton, and her colleagues focused on fountain grass (Cenchrus squamulatus) — a natural apomict — which looked like a good candidate for containing an apomixis gene5. The gene they identified was similar to BABY BOOM, which can provoke plant tissue to spontaneously form embryos. But it took Ozias-Akins’s group almost a decade to confirm their hunch about the gene’s role in apomixis. They introduced the fountain grass gene into pearl millet (Pennisetum glaucum) — which reproduces sexually — and discovered that embryos spontaneously developed without fertilization6. “That was pretty exciting,” says Ozias-Akins.

An international team of researchers has created a type of sorghum that can reproduce asexually and will be testing it soon in a field trial in Australia.Credit: Getty
Meanwhile, Sundaresan was zeroing in on BABY BOOM from an entirely different direction. “My lab really was not working on apomixis at all,” he says. Instead, his group was looking for genes that are active during the crucial transition from an unfertilized egg cell to a fertilized one that can develop into an embryo.
Working with rice, Sundaresan’s team extracted RNA — the read-out product of active genes — from individual egg cells in the hours immediately following pollination. They found that BABY BOOM RNA was abundant. Sundaresan’s group went on to show that BABY BOOM was acting as a trigger for embryogenesis, and the active copy of the gene was delivered to the egg cell by the sperm7. Next, Sundaresan and his colleague Imtiyaz Khanday, an agronomist at the University of California, Davis, showed that the sperm wasn’t required at all. They inserted a copy of the BABY BOOM gene with instructions to switch on in the egg cell — where its own copy of the gene is silenced. With this technique, the team set embryogenesis in motion8.
Putting it all together
After Sundaresan learnt about the MiMe rice work in 2016, he contacted Mercier to collaborate on combining MiMe and BABY BOOM. They edited the MiMe genes in rice containing egg-cell-active BABY BOOM. In doing so, they achieved the long-sought goal of the field: an apomictic plant that propagates clones of itself8. Although the efficiency was poor — only 10–30% of seeds were clonal — the work provided a proof-of-concept for combining the two essential elements of apomixis in a valuable crop species.
Fungi bacon and insect burgers: a guide to the proteins of the future





