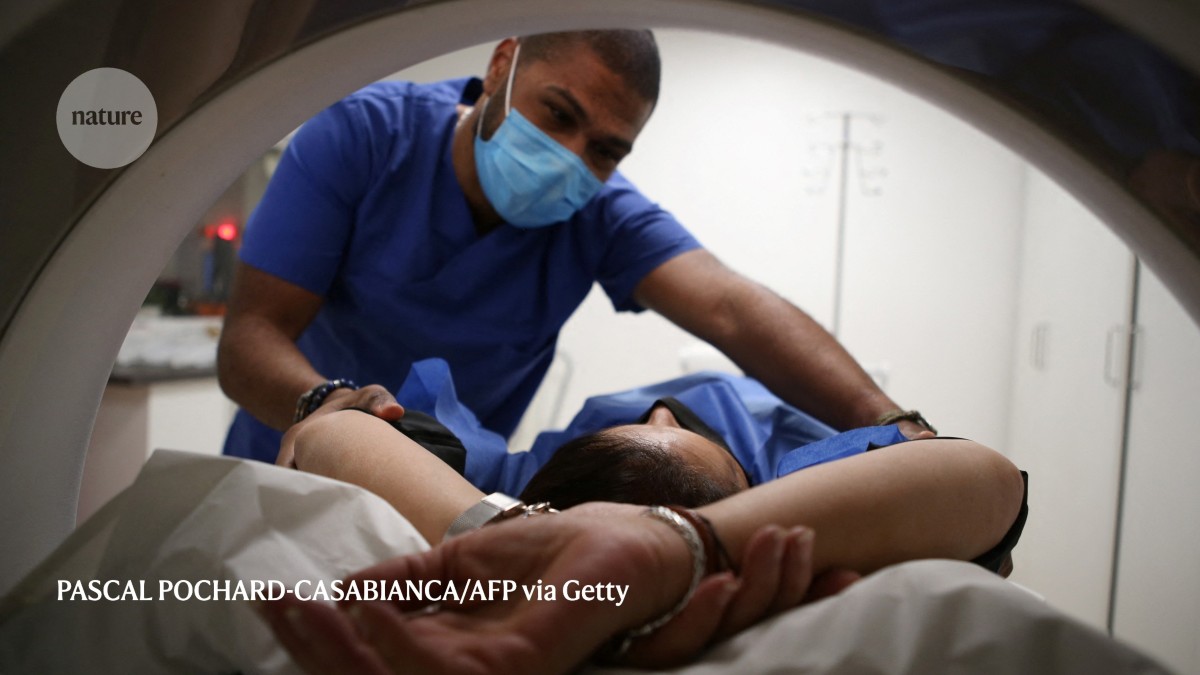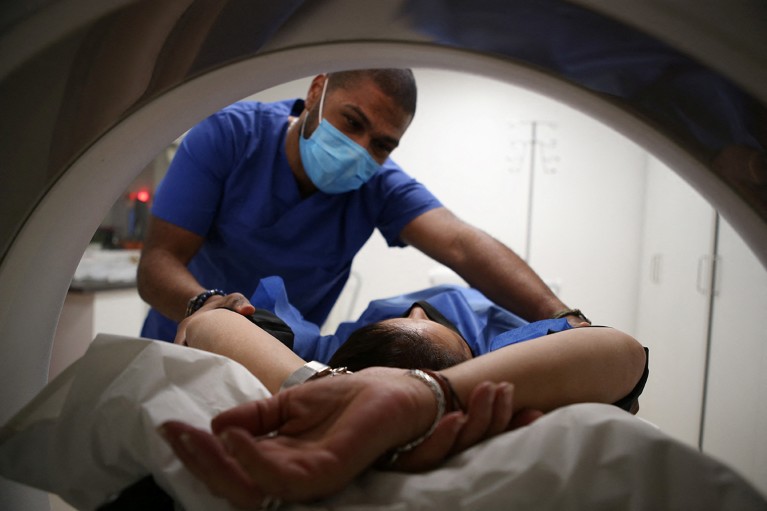
Screening methods, such as MRI, are inaccessible to many populations, prompting a need for cheaper, large-scale programmes.Credit: Pascal Pochard-Casabianca/AFP via Getty
There is no ‘good’ time for a cancer diagnosis. But catching it early generally offers the best opportunity to treat it effectively — or even curatively. The impact of early-detection screening strategies for several relatively common malignancies, including mammography for breast cancer and low-dose computed tomography (LDCT) for lung cancer, has been substantial, according to David Crosby, who heads the Prevention and Early Detection Research programme at Cancer Research UK (CRUK). “We know from large-scale clinical trials that breast, colorectal, cervical and lung cancer screening works,” says Crosby. Nearly 5 million cancer-related deaths were averted in the United States alone between 1975 and 2020 as a result of screening programmes and cancer prevention efforts such as anti-tobacco campaigns, for example, according to estimates from a 2024 study1 led by researchers at the US National Cancer Institute.
Nature Index 2025 Cancer
Many cancers, however, pose challenges for large-scale screening programmes because they are either rare or occur in organs that are difficult to monitor without expensive or invasive procedures. Some cancers, such as glioblastoma — an aggressive and fast-growing type of brain tumour — tick both boxes. Even for cancers that are amenable to early detection, many patients are overlooked, because of limited understanding of the potential risk factors. “There’s tremendous concern right now about cancer in the younger population being on the rise, but they’re not eligible for screening,” says Samir Hanash, a cancer prevention specialist at the MD Anderson Cancer Center in Houston, Texas.
There are also substantial disparities in screening access between rich and poor populations. “Cervical cancer is the most preventable cancer, yet hundreds of thousands of women die every year from it,” says Rebecca Richards-Kortum, a bioengineer at Rice University, in Houston. Data from the World Health Organization show that 94% of cervical cancer deaths occur in low- and middle-income countries.
The good news is that solutions are emerging that could help clinicians intercept a broader range of tumours earlier and more accurately. “I think we’re seeing an explosion of technological progress and biological insight, which is opening doors for a new generation of much more targeted and precise screening,” says Crosby.
Tumour targeting
Imaging-based screening methods such as mammography and LDCT can offer a direct glimpse of a tumour. But many cancers remain hard to spot until they reach a relatively advanced — and typically, more lethal — stage. This problem has spurred research into liquid biopsy tests, in which blood or other body fluids are sampled to detect molecular signatures, or ‘biomarkers’, that herald the emergence of a malignant growth.
The challenge is finding the right biomarker. Crosby highlights the long-standing debate over the use of elevated levels of the prostate-specific antigen (PSA) protein in the blood as early warning for prostate cancer. “Numerous trials have shown that there is or can be a mortality benefit from PSA screening,” he says, “but you will also have a very, very large proportion of false positives.” PSA levels can also peak in response to infection or inflammation of the prostate, for example, leading to needless biopsies that can impair sexual or urinary function.
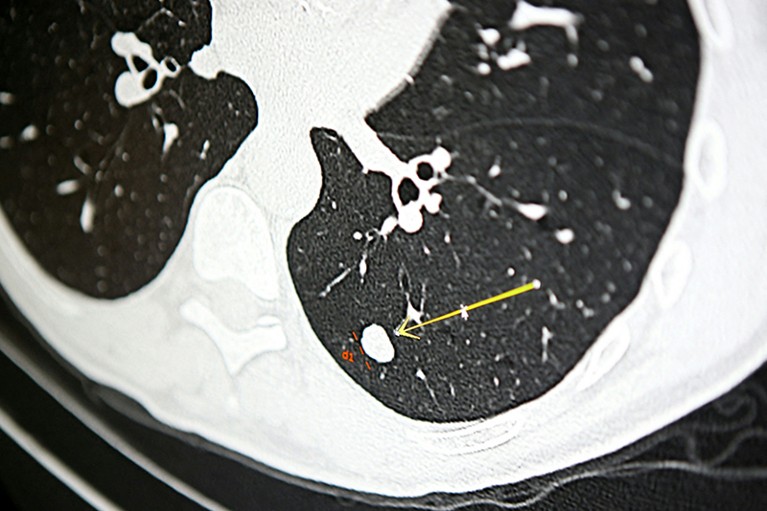
A nodule on a scan for lung cancer, screening for which is based on smoking history.Credit: Pascal Pochard-Casabianca/AFP via Getty
Researchers need to look for more signs of early-stage cancer using multiple biomarkers, says Catherine Alix-Panabières, an oncologist at the University of Montpellier in France. “My belief is that we need to combine,” she says. “If one circulating biomarker should be enough, we should have found it already.”
Tumour-specific proteins are one potential target, and researchers are investigating other biomarkers ranging from DNA shed by dead tumour cells to more indirect indicators of a patient’s immune response to tumour formation. Alix-Panabières is leading the French PANLIPSY research consortium, which aims to identify molecular features present in patients with pancreatic cancer, but not healthy controls, with the goal of developing a simple blood-based test. “We will use artificial intelligence [AI] to see what is really discriminating or distinguishing healthy donors or benign diseases of the pancreas from cancer patients,” says Alix-Panabières.
Hanash is coordinating a similar effort for lung and other cancers, combing through hundreds of thousands of biobank specimens to uncover biomarker patterns that emerge in asymptomatic people who eventually develop tumours. In 2022, Hanash and colleagues showed2 that a test combining four different protein biomarkers with an algorithmic risk-prediction model enabled screening-based detection of 9% more lung cancer cases in people with a history of heavy smoking, while reducing unnecessary screening by 13%.
With the right combination of biomarkers, one can potentially intercept many different cancers from a single blood sample. Several companies are now developing such multi-cancer early detection (MCED) assays. For example, the Galleri MCED test, developed by California-based health-care company GRAIL, can reportedly detect more than 50 different cancers3. The test analyses patterns of chemical changes on tumour-derived DNA in the blood to reveal both the presence of a tumour and its location in the body. In 2023, the company published results from the Pathfinder clinical trial4, in which it screened 6,621 apparently healthy individuals and caught 29 previously undetected cancers in the pancreas, intestine, breast and other tissues. Galleri is now undergoing a randomized controlled trial in the United Kingdom, and is already commercially available as a prescribed laboratory test in the United States.
Crosby points out that the majority of those 29 tumours were relatively advanced. “It looks like, by and large, [MCEDs] are not particularly sensitive for stage one cancers, which are the ones you want to find,” he says. It also remains unclear how many tumour types are realistically detectable from a single blood sample. Alix-Panabières notes that the ability to detect a given tumour early on can be influenced by the type of biomarker that’s being evaluated and the site of blood collection. Getting all of this right with a single sample becomes a big ask. “It would be nice to be more focused on one cancer type and be able to detect this cancer type early enough to act and to cure the patient,” she says.
Rethinking risk
Like other screening strategies, liquid biopsy tests must strike a balance between enabling sensitive detection of true cancer cases while minimizing false positives and unnecessary diagnostic procedures. Galleri performed well in this regard, with less than 1% of the Pathfinder participants receiving a false-positive result. But this would produce too many misleading ‘hits’ if scaled out to the entire population. For some cancers, incidence can be so low that false-positives quickly dominate. For example, pancreatic cancer has a global incidence of 5 cases per 100,000 people, such that a test with a 1% false-positive rate would flag 200 healthy people for every cancer caught.
“Many of our screening tests that have been most successful are personalized in some way,” says Lecia Sequist, a medical oncologist at the Massachusetts General Hospital in Boston. She cites cervical cancer, where testing for certain subtypes of human papillomavirus (HPV) can identify women who should be prioritized for screening. But risks for other cancers are typically based on broad considerations such as age, or smoking history in the case of lung-cancer screening, which has led some clinicians to reassess the utility of such well-established risk factors. “We’re getting all different kinds of lung cancers that come up in people who haven’t smoked,” says Sequist. “My sense is that for lung cancer and many other cancers, there are actually a lot of risk factors that we don’t know in our patients.”
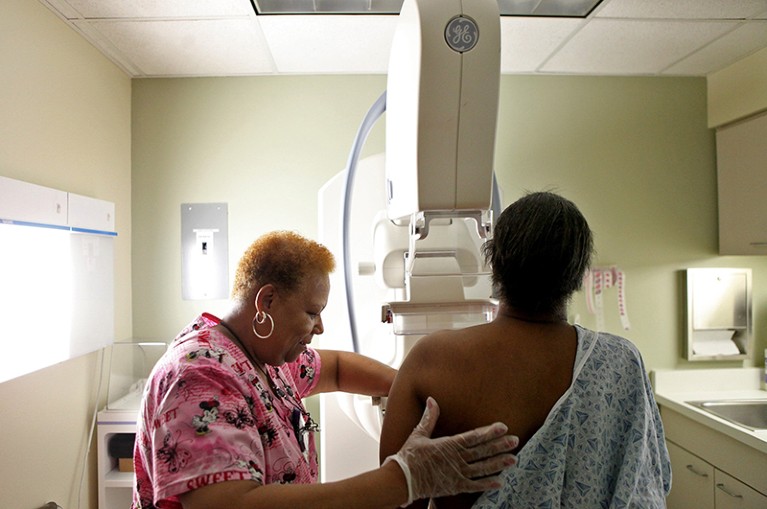
Mammography is being made more efficient through the use of AI in reading results.Credit: Heather Charles/TNS via ZUMA Press Wire/Shutterstock