Canales, A., Park, S., Kilias, A. & Anikeeva, P. Multifunctional fibers as tools for neuroscience and neuroengineering. Acc. Chem. Res. 51, 829–838 (2018).
Zhang, Y. et al. Multifunctional fibers to shape future biomedical devices. Adv. Funct. Mater. 29, 1902834 (2019).
Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160 (2019).
Kahrilas, P. J. & Sifrim, D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 135, 756–769 (2008).
Zeng, K., Shi, X., Tang, C., Liu, T. & Peng, H. Design, fabrication and assembly considerations for electronic systems made of fibre devices. Nat. Rev. Mater. 8, 552–561 (2023).
Richard, I., Schyrr, B., Aiassa, S., Carrara, S. & Sorin, F. All-in-fiber electrochemical sensing. ACS Appl. Mater. Interfaces 13, 43356–43363 (2021).
Kim, I. H. et al. Human-muscle-inspired single fibre actuator with reversible percolation. Nat. Nanotechnol. 17, 1198–1205 (2022).
Hwang, S. et al. Integration of multiple electronic components on a microfibre towards an emerging electronic textile platform. Nat. Commun. 13, 3173 (2022).
Ding, T. et al. Scalable thermoelectric fibers for multifunctional textile-electronics. Nat. Commun. 11, 6006 (2020).
Rein, M. et al. Diode fibres for fabric-based optical communications. Nature 560, 214–218 (2018).
Kanik, M. et al. Strain-programmable fiber-based artificial muscle. Science 365, 145–150 (2019).
Sahasrabudhe, A. et al. Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits. Nat. Biotechnol. 42, 892–904 (2023).
Xu, Z. & Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2, 571 (2011).
Zhang, S. et al. Biomimetic spinning of soft functional fibres via spontaneous phase separation. Nat. Electron. 6, 338–348 (2023).
Pi, Q. et al. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv. Mater. 30, 1706913 (2018).
Nan, K. et al. Low-cost gastrointestinal manometry via silicone–liquid-metal pressure transducers resembling a quipu. Nat. Biomed. Eng. 6, 1092–1104 (2022).
Lee, J. et al. Stretchable and suturable fibre sensors for wireless monitoring of connective tissue strain. Nat. Electron. 4, 291–301 (2021).
Kalidasan, V. et al. Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nat. Biomed. Eng. 5, 1217–1227 (2021).
Qu, Y. et al. Superelastic multimaterial electronic and photonic fibers and devices via thermal drawing. Adv. Mater. 30, 1707251 (2018).
Driscoll, N. et al. Multifunctional neural probes enable bidirectional electrical, optical, and chemical recording and stimulation in vivo. Adv. Mater. https://doi.org/10.1002/adma.202408154 (2024).
Lee, Y. et al. Selectively micro-patternable fibers via in-fiber photolithography. ACS Cent. Sci. 6, 2319–2325 (2020).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Tian, Y. et al. An ultraflexible electrode array for large‐scale chronic recording in the nonhuman primate brain. Adv. Sci. 10, 2302333 (2023).
Le Floch, P. et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2024).
Rivkin, B. et al. Electronically integrated microcatheters based on self-assembling polymer films. Sci. Adv. 7, eabl5408 (2021).
Wang, S. et al. A self-assembled implantable microtubular pacemaker for wireless cardiac electrotherapy. Sci. Adv. 9, eadj0540 (2023).
Huang, W. et al. Monolithic mtesla-level magnetic induction by self-rolled-up membrane technology. Sci. Adv. 6, eaay4508 (2020).
Gabler, F., Karnaushenko, D. D., Karnaushenko, D. & Schmidt, O. G. Magnetic origami creates high performance micro devices. Nat. Commun. 10, 3013 (2019).
Lipomi, D. J., Chiechi, R. C., Reus, W. F. & Whitesides, G. M. Laterally ordered bulk heterojunction of conjugated polymers: nanoskiving a jelly roll. Adv. Funct. Mater. 18, 3469–3477 (2008).
Ruijie, X. et al. Rolling 2D bioelectronic film into 1D: a suturable long-term implantable soft microfiber. Preprint at bioRxiv https://doi.org/10.1002/adma.202408154 (2023).
Liu, Y. et al. A high-density 1,024-channel probe for brain-wide recordings in non-human primates. Nat. Neurosci. 27, 1620–1631 (2024).
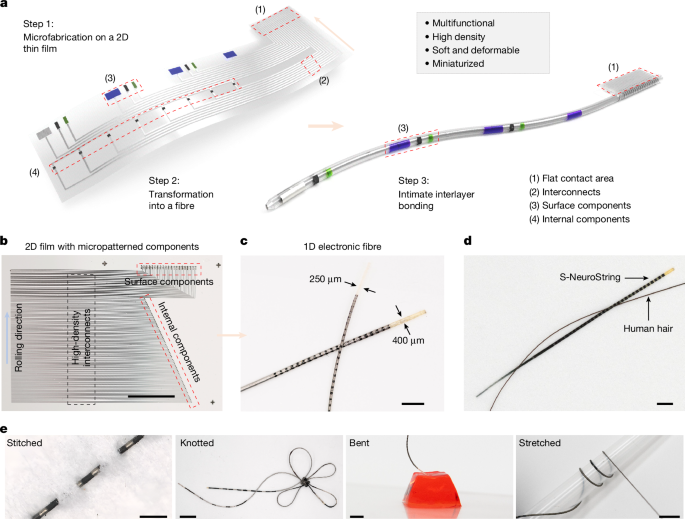
Khatib, M. et al. Spiral NeuroString: high-density soft bioelectronic fibers for multimodal sensing and stimulation. Preprint at bioRxiv https://doi.org/10.1101/2023.10.02.560482 (2023).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Li, J. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606, 94–101 (2022).
Nan, K. et al. Mucosa-interfacing electronics. Nat. Rev. Mater. 7, 908–925 (2022).
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2019).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Pannala, R. et al. Devices for esophageal function testing. VideoGIE 7, 1–20 (2022).
Scholz, S., Sood, V. & Sharbaugh, E. in The SAGES Manual of Physiologic Evaluation of Foregut Diseases (eds Patel, A. D. et al.) 591–624 (Springer, 2023).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Salimi-Jazi, F. et al. Perioperative gastrointestinal myoelectric activity measurement using wireless external patches. J. Surg. Res. 302, 186–199 (2024).
Dubrovsky, G. et al. Intestinal electrical stimulation to increase the rate of peristalsis. J. Surg. Res. 236, 153–158 (2019).
Southwell, B. R. Electro-neuromodulation for colonic disorders—review of meta-analyses, systematic reviews, and RCTs. Neuromodulation 23, 1061–1081 (2020).
Liu, J. et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl Acad. Sci. USA 117, 14769–14778 (2020).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Zhou, T. et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 22, 895–902 (2023).
Tchoe, Y. et al. Human brain mapping with multithousand-channel PtNRGrids resolves spatiotemporal dynamics. Sci. Transl. Med. 14, eabj1441 (2022).
Viswam, V., Obien, M. E. J., Franke, F., Frey, U. & Hierlemann, A. Optimal electrode size for multi-scale extracellular-potential recording from neuronal assemblies. Front. Neurosci. 13, 385 (2019).
Boehler, C., Carli, S., Fadiga, L., Stieglitz, T. & Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15, 3557–3578 (2020).
Zhao, E. T. et al. A CMOS-based highly scalable flexible neural electrode interface. Sci. Adv. 9, eadf9524 (2023).
Won, C. et al. Mechanically tissue‐like and highly conductive au nanoparticles embedded elastomeric fiber electrodes of brain–machine interfaces for chronic in vivo brain neural recording. Adv. Funct. Mater. 32, 2205145 (2022).
Le Floch, P. et al. 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers. Nat. Nanotechnol. 19, 319–329 (2023).
Wang, W. et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 380, 735–742 (2023).
Du, Z. J. et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 53, 46–58 (2017).
Joo, H. R. & Frank, L. M. The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci. 19, 744–757 (2018).
Kim, H. D., Huh, J. H., Kim, E. Y. & Park, C. C. Comparison of properties of thermoplastic polyurethane elastomers with two different soft segments. J. Appl. Polym. Sci. 69, 1349–1355 (1998).
Lin, J. et al. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 5, 5714 (2014).
Hashemi, P., Dankoski, E. C., Petrovic, J., Keithley, R. B. & Wightman, R. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal. Chem. 81, 9462–9471 (2009).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Khatib, M., Zohar, O., Saliba, W. & Haick, H. A multifunctional electronic skin empowered with damage mapping and autonomic acceleration of self‐healing in designated locations. Adv. Mater. 32, 2000246 (2020).
Guinovart, T., Crespo, G. A., Rius, F. X. & Andrade, F. J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 821, 72–80 (2014).
Oh, S. Y. et al. Skin-attachable, stretchable electrochemical sweat sensor for glucose and pH detection. ACS Appl. Mater. Interfaces 10, 13729–13740 (2018).
Swaminathan, M. et al. Video imaging and spatiotemporal maps to analyze gastrointestinal motility in mice. J. Vis. Exp. 108, e53828 (2016).
Patel, B. Electroanalytical approaches to study signaling mechanisms in the gastrointestinal tract. Neurogastroenterol. Motility 23, 595–605 (2011).
Marcelli, G. & Patel, B. A. Understanding changes in uptake and release of serotonin from gastrointestinal tissue using a novel electroanalytical approach. Analyst 135, 2340–2347 (2010).
Pachitariu, M., Steinmetz, N. A., Kadir, S. N., Carandini, M. & Harris, K. D. Fast and accurate spike sorting of high-channel count probes with KiloSort. Adv. Neural Inf. Process. Syst. 29, 2199 (2016).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. eLife 9, e61834 (2020).
Hill, D. N., Mehta, S. B. & Kleinfeld, D. Quality metrics to accompany spike sorting of extracellular signals. J. Neurosci. 31, 8699–8705 (2011).
Gonzalez, A. & Giocomo, L. M. Parahippocampal neurons encode task-relevant information for goal-directed navigation. eLife 12, RP85646 (2024).
Jones, E. A., Gillespie, A. K., Yoon, S. Y., Frank, L. M. & Huang, Y. Early hippocampal sharp-wave ripple deficits predict later learning and memory impairments in an Alzheimer’s disease mouse model. Cell Rep. 29, 2123–2133.e2124 (2019).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Wang, L. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 4, 159–171 (2020).
Kessing, B. F., Weijenborg, P. W., Smout, A. J., Hillenius, S. & Bredenoord, A. J. Water-perfused esophageal high-resolution manometry: normal values and validation. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G491–G495 (2014).
Wang, K., Duan, L.-p., Ge, Y., Xia, Z.-w. & Xu, Z.-j. A comparative study of 22-channel water-perfusion system and solid-state system with 36-sensors in esophageal manometry. BMC Gastroenterol. 12, 157 (2012).
Liem, O. et al. Solid‐state vs water‐perfused catheters to measure colonic high‐amplitude propagating contractions. Neurogastroenterol. Motility 24, 345–e167 (2012).
Rasijeff, A. M., Withers, M., Burke, J. M., Jackson, W. & Scott, S. M. High‐resolution anorectal manometry: a comparison of solid‐state and water‐perfused catheters. Neurogastroenterol. Motility 29, e13124 (2017).
Koppen, I. et al. Motility of the left colon in children and adolescents with functional constpation; a retrospective comparison between solid‐state and water‐perfused colonic manometry. Neurogastroenterol. Motility 30, e13401 (2018).
de Leon, A., Thörn, S.-E. & Wattwil, M. High-resolution solid-state manometry of the upper and lower esophageal sphincters during anesthesia induction: a comparison between obese and non-obese patients. Anesth. Analg. 111, 149–153 (2010).
Corsetti, M. et al. Pan-colonic pressurizations associated with relaxation of the anal sphincter in health and disease: a new colonic motor pattern identified using high-resolution manometry. Am. J. Gastroenterol. 112, 479–489 (2017).
Lee, T. H. & Bharucha, A. E. How to perform and interpret a high-resolution anorectal manometry test. J. Neurogastroenterol. Motility 22, 46 (2016).
Arkwright, J. et al. In-vivo demonstration of a high resolution optical fiber manometry catheter for diagnosis of gastrointestinal motility disorders. Opt. Express 17, 4500–4508 (2009).
Arkwright, J. W. et al. Design of a high-sensor count fibre optic manometry catheter for in-vivo colonic diagnostics. Opt. Express 17, 22423–22431 (2009).
Dinning, P. et al. Low‐resolution colonic manometry leads to a gross misinterpretation of the frequency and polarity of propagating sequences: initial results from fiber‐optic high‐resolution manometry studies. Neurogastroenterol. Motility 25, e640–e649 (2013).
Dinning, P. et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high‐resolution fiber‐optic manometry. Neurogastroenterol. Motility 26, 1443–1457 (2014).
Racz, R. R. et al. jULIEs: nanostructured polytrodes for low traumatic extracellular recordings and stimulation in the mammalian brain. J. Neural Eng. 19, 016041 (2022).
Lee, S. H. et al. Scalable thousand channel penetrating microneedle arrays on flex for multimodal and large area coverage brainmachine interfaces. Adv. Funct. Mater. 32, 2112045 (2022).
Raducanu, B. C. et al. Time multiplexed active neural probe with 1356 parallel recording sites. Sensors 17, 2388 (2017).
Woeppel, K. et al. Explant analysis of Utah electrode arrays implanted in human cortex for brain–computer-interfaces. Front. Bioeng. Biotechnol. 9, 1137 (2021).
Obaid, A. et al. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 6, eaay2789 (2020).
Sahasrabuddhe, K. et al. The Argo: a high channel count recording system for neural recording in vivo. J. Neural Eng. 18, 015002 (2021).
Patel, P. R. et al. Chronic in vivo stability assessment of carbon fiber microelectrode arrays. J. Neural Eng. 13, 066002 (2016).
Patel, P. R. et al. High density carbon fiber arrays for chronic electrophysiology, fast scan cyclic voltammetry, and correlative anatomy. J. Neural Eng. 17, 056029 (2020).
Wei, X. et al. Nanofabricated ultraflexible electrode arrays for high‐density intracortical recording. Adv. Sci. 5, 1700625 (2018).
Zhao, S. et al. Tracking neural activity from the same cells during the entire adult life of mice. Nat. Neurosci. 26, 696–710 (2023).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Zhao, Z. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng. 7, 520–532 (2023).
Middya, S. et al. Multishank thin‐film neural probes and implantation system for high‐resolution neural recording applications. Adv. Electron. Mater. 9, 2200883 (2022).
Park, S. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat. Commun. 12, 3435 (2021).
Lee, K. et al. Flexible, scalable, high channel count stereo-electrode for recording in the human brain. Nat. Commun. 15, 218 (2024).
Guan, S. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 5, eaav2842 (2019).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31.e25 (2019).
Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 21, e16194 (2019).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).