Generation of endogenously tagged DHR1, KRE33, UTP7 and UTP14 strains
Assembly intermediates of the S. cerevisiae SSU processomes were purified from three genetically modified BY4741 (MATa his3Δ leu2Δ0 met15Δ0 ura3Δ0) strains. Where genes of interest (DHR1, KRE33, UTP7 and UTP14) were endogenously tagged: the first strain containing C-terminal tandem 3C protease-cleavable mCherry and TEV-cleavable alfa peptide tag on DHR1 along with a 3C cleavable C-terminal GFP-tag on KRE33 (MATa his3Δ leu2Δ0 met15Δ0 ura3Δ0 DHR1-linker-tev-alfa tag-3c-mCherry under hygromycin B selection and KRE33-linker-tev-GFP under nourseothricin selection), the second strain containing only C-terminal tandem 3C protease-cleavable mCherry and TEV-cleavable alfa peptide tag on DHR1 (MATa his3Δ leu2Δ0 met15Δ0 ura3Δ0 DHR1-linker-tev-alfa tag-3c-mCherry under hygromycin B selection) and the third strain containing C-terminal tandem 3C protease-cleavable mCherry and a TEV-cleavable alfa peptide tag on DHR1 along with TEV-cleavable C-terminal GFP-tag on UTP7 and C-terminally streptavidin-binding-peptide (sbp) tag on UTP14 (MATa his3Δ leu2Δ0 met15Δ0 ura3Δ0 DHR1-linker-tev-alfa tag-3c-mCherry under hygromycin B selection and UTP7-linker-tev-GFP under G418 selection and UTP14-linker-SBP under nourseothricin selection). The three strains were generated with standard genomic tagging techniques using the following primers for yeast genomic tagging: DHR1 forward primer, TCACCAGAAAGGGCTTCCAGACCATCACAGGTGAAGAGAAAGAAAAAAAAACGCTGCAGGTCGACGGATCC; DHR1 reverse primer, TTAAGTGGTTGCAATTATTTGATGCCCTAGATAGGAATAATGTATTCTTCGGCAGATCCGCGGCCGCATAGG; Kre33 forward primer, AAGAGATGAAAGCTATGAAAAAACCAAGAAAGTCTAAAAAGGCTGCAAATACGCTGCAGGTCGACGGATCC; KRE33 reverse primer, TGTAAAGGTTCAAACATCAACTATGTTTCTATTCTATATTATTGTACAAAGGCAGATCCGCGGCCGCATAGG; UTP7 forward primer, TTTCAGAAGACCACAAGGATGTCATCGAAGAGGCATTGAGCAGATTCGGCACGCTGCAGGTCGACGGATCC; UTP7 reverse primer, GCTATATTAATATGCAATCGATTCTCATACTGTCAACTTTTTGAACATGAGGCAGATCCGCGGCCGCATAGG; UTP14 forward primer, TCATGACTAAGCCAGGCCAAGTTATTGATCCTTTGAAGGCACCATTTAAGACGCTGCAGGTCGACGGATCC; UTP14 reverse primer, ATTATTCCAGTATTACTATTCTACACAATGCATAATAAATAGATATAAAAGGCAGATCCGCGGCCGCATAGG.
The 3 strains were grown at 30 °C in YPD medium (1% yeast extract, 2% peptone and 2% glucose) to optical density of OD 1 measured at 600 nm. Cells were harvested at 4,000g for 5 min, washed once with 1 l of ice cold ddH2O and once with a volume of ddH2O supplemented with protease inhibitors (E64, pepstatin and PMSF) equal to the mass of the cell pellet. The final pellets of ~30–40 g were flash frozen in liquid nitrogen. Pellets were lysed by cryo-grinding using a Retsch Planetary Ball Mill PM100 and powder was stored at –80 °C.
Purification of SSU processome intermediates
Cryo-ground powder was resuspended with buffer 1 (60 mM Tris pH7.5, 50 mM NaCl, 2 mM MgCl2, 5% glycerol, 0.1% NP-40) with addition of PMSF, pepstatin and E64 protease inhibitors. The suspension was cleared by centrifugation at 4 °C and 40,000g for 20 min and lysate was incubated with 800 µl of packed NHS–Sepharose beads (Cytiva) coupled to anti-mCherry nanobodies for the first capture for all purifications and incubated for 3.5 h at 4 °C on a nutator. Beads were pelleted by centrifugation at 4 °C for 1 min at 127g. After five washes in buffer 1, complexes were eluted in buffer EB (60 mM Tris, 50 mM NaCl, 5 mM MgCl2, 2% glycerol, 0.01% NP-40) supplemented with 3C protease for 1 h at 4 °C. Beads were pelleted by centrifugation at 4 °C for 1 min at 127g and supernatant was eluted and incubated with 80 µl of packed NHS–Sepharose beads (Cytiva) coupled to anti-GFP nanobodies for second capture (Dhr1–Kre33 and Dhr1–Utp14 purifications). Anti-alfa nanobodies were used for a second Dhr1 (Dhr1 only). Beads were incubated for 1 h at 4 °C on a nutator and pelleted by centrifugation at 4 °C for 1 min at 127g and washed twice with 2 ml of Buffer EB. After the second wash, for Dhr1–Kre33 and Dhr1 only purifications, beads were pelleted and resuspended with 25 µl of Buffer EB supplemented with 1 mM DTT and TEV protease and incubated on ice for 1 h. Beads were pelleted by centrifugation at 4 °C for 10 min at 21,130g and the supernatant was collected. For Dhr1–Utp14 purification, after the second capture (here Utp7), beads were pelleted by centrifugation at 4 °C for 1 min at 127g and flowthrough was collected, the rest of the beads were discarded (this step was used to remove earlier contaminating complexes). The flowthrough was incubated with 40 µl of packed NHS–Sepharose beads (Cytiva) coupled to streptavidin for 1 h at 4 °C on a nutator. Beads were pelleted by centrifugation at 4 °C for 1 min at 127g and washed twice with 2 ml of Buffer EB. After the second wash, beads were pelleted and resuspended with 40 µl of Buffer EB supplemented with 1 mM DTT and 5 mM of d-biotin (Amresco) and incubated on ice for 20 min. Beads were pelleted by centrifugation at 4 °C for 10 min at 21,130g and the supernatant was collected.
Cryo-EM grid preparation and data acquisition
Cryo-EM grids were prepared using a Vitrobot Mark IV robot (FEI Company) set to 90% humidity and 18 °C temperature. Three and a half microlitres of the eluted solution was applied to a glow-discharged Quantifoil Au R3.5/1 with a layer of 2-nm ultrathin carbon (LFH7100AR35, Electron Microscopy Sciences). After 2.5 min incubation inside the Vitrobot chamber, the excess solution was manually blotted and a fresh sample of 3.5 µl was reapplied inside the Vitrobot chamber and incubated for another 2.5 min. The lower the concentration of particles of a given preparation, the higher the number of applications that were done on each grid. For the Dhr1 and Kre33 dataset, a total of five applications were done on each grid. The grid was then blotted (blot force of 8 and blot time of 9 s) and plunged into liquid ethane. For the Dhr1 dataset, a total of two applications were done for each grid. The grid was then blotted (blot force of 8 and blot time of 7 s) and plunged into liquid ethane and for the Dhr1 and Utp14 dataset, a total of 5 applications were done on each grid to achieve a good distribution of particles. The grid was then blotted (blot force of 8 and blot time of 9 s) and plunged into liquid ethane. Grids were imaged on a Titan Krios electron microscope (FEI) with an energy filter (slit width of 20 eV) and a K3 Summit detector (Gatan) operating at 300 kV with a nominal magnification of 64,000×.
SerialEM30 was used to collect four datasets. A total of 44,272 micrographs was collected for the Kre33–Dhr1 dataset, two datasets of Dhr1 were collected totalling 84,463 micrographs and 85,031 micrographs were collected for the Dhr1 and Utp14 dataset. All datasets were collected with a defocus range of −1 to −2.5 µm and a super-resolution pixel size of 0.54 Å. Micrographs contained 40 frames using a total dose of 25.3–30.8 e− pixel−1 s−1 (specimen pixel size of 1.08 Å per pixel) with an exposure time of 2–2.5 s and a total dose of 61.7–63.1e− Å−2. A multi-shot strategy was used to record nine micrographs per hole at each stage position with the same defocus range, electron dose and frame count.
Cryo-EM data processing Dhr1 and Kre33 dataset
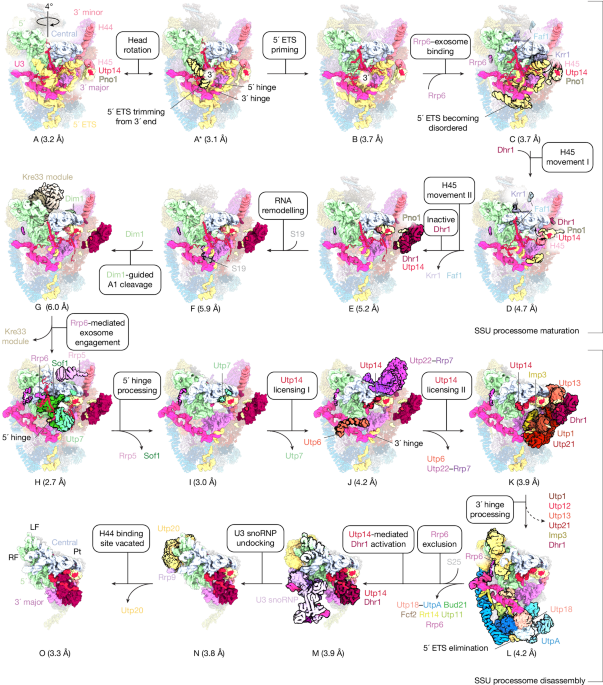
The Dhr1 and Kre33 dataset was processed using a combination of RELION 5beta31 and cryoSparc32 v.4.6. A total of 44,272 movies was gain corrected, dose weighted and aligned, with each dataset having different optic groups and binned to a pixel size of 1.08 Å using RELION’s implementation of a MotionCor2-like algorithm33. Micrograph defocus was estimated using Gctf34. Particles were picked using Laplacian (autopick) in RELION 5beta and post 2D classification a total of 2,817,609 particles were re-extracted at a pixel size of 4.32 Å per pixel (4× binning) and underwent 3D classification in RELION 5beta with alignment using a reference map from previous datasets. Two good classes were combined, and duplicates were removed to result in 308,351 total particles. These particles were subjected to three rounds of contrast transfer function (CTF) refinement and Bayesian polishing in RELION 5beta. Post polishing all homogenous refinements were completed in CryoSparc v.4.6 and classifications were all done in RELION 5beta, particle positions from cryosparc were converted into RELION using pyem software csparc2star.py35. The polished particles were subjected to a homogenous refinements resulting in a reconstruction at a global resolution of 3.3 Å. To separate the states present in the consensus reconstruction, multiple 3D classifications without alignment were performed. This was followed by 3D variability36 in CryoSparc v.4.6 for analysis of the type of heterogeneity present in the data which guided the creation of a mask around the region of interest and further 3D classification without alignment on the region of variability. Eight distinct states (states A–G) were isolated from the dataset that showed unique features in the progression of maturation of the SSU processome pathway (Supplementary Fig. 1). The global resolution of the eight states ranged from 3–5.9 Å.
Cryo-EM data processing Dhr1 dataset
The Dhr1 dataset was processed using a combination of RELION 5beta31 and cryoSparc32 v.4.6. A total of 84,463 movies was gain corrected, dose weighted and aligned, with each dataset having different optic groups and binned to a pixel size of 1.08 Å using RELION’s implementation of a MotionCor2-like algorithm33. Micrograph defocus was estimated using Gctf34. Particles were picked using crYOLO 1.7.537 and post 2D classification a total of 13,198,550 particles were re-extracted at a pixel size of 4.32 Å per pixel (4× binning) and underwent heterogenous refinement in CryoSparc v.4.6 using a reference map from previous classification in RELION 5beta. One good class was isolated resulting in 1,933,969 total particles. These particles were subjected to three rounds of CTF refinement and Bayesian polishing in RELION 5beta. Post polishing all homogenous refinements were completed in CryoSparc v.4.6 and classifications were all done in RELION 5beta. Particle positions from cryosparc were converted into RELION using pyem software csparc2star.py35. The polished particles were subjected to a homogenous refinement resulting in a reconstruction at a global resolution of 3 Å. To separate the states present in the consensus reconstruction, multiple iterations of 3D classifications without alignment was performed. This was followed by 3D variability36 in CryoSparc v.4.6 for analysis of the type of heterogeneity present in the data which guided the creation of a mask around the region of interest and further 3D classification without alignment on the region of variability. Seven distinct states (states H–N) were isolated from the dataset that showed unique features in the progression of the disassembly of the SSU processome pathway (Supplementary Fig. 2). The global resolution of the seven states ranged from 2.65 to 4.3 Å.
Cryo-EM data processing Dhr1 and Utp14 dataset
The Dhr1 and Utp14 dataset was processed using a combination of RELION 5beta31 and cryoSparc32 v.4.6. 85,031 movies were gain corrected, dose weighted, aligned, with each dataset having different optic groups and binned to a pixel size of 1.08 Å using CryoSparc v 4.6 motion correction. Micrograph defocus was estimated using Patch CTF and particles were picked using a template picker resulting in a total of 27,069,273 particles which underwent heterogenous refinement and subsequent global and local CTF refinements, followed by reference motion correction. Post polishing all homogenous refinements were completed in CryoSparc v.4.6 and classifications were all done in RELION 5beta. Particle positions from cryosparc were converted into RELION using pyem software csparc2star.py35. The polished particles were subjected to a homogenous refinement resulting in a reconstruction at a global resolution of 2.9 Å. To separate the states present in the consensus reconstruction, multiple iterations of 3D classifications without alignment were performed. This was followed by 3D variability36 in CryoSparc v.4.6 for analysis of the type of heterogeneity present in the data which guided the creation of a mask around the region of interest and further 3D classification without alignment on the region of variability. state O was isolated from the dataset with global resolution of 3.25 Å that showed clear density for Utp14-bound Dhr1 (Supplementary Fig. 3).
Generation of focused and composite maps for model buildings
Composite maps for the total of 16 states were generated from combined focused maps to facilitate model building. Focused maps were generated using subtraction and refinement masks generated in CryoSparc v.4.6. Each focused map was made by particle subtraction with a masked region followed by masked local refinement. Local resolution estimation for overall and all focused maps and filtering of the overall map were performed using CryoSparc v.4.6. Focused maps were combined into a composite map using the ‘vop max’ command in ChimeraX38 (Supplementary Figs. 4–25).
Model building and refinement
A combination of AlphaFold structure predictions39, existing X-ray/EM structures, and de novo model building was used to build the 16 SSU processome assembly intermediates. A starting model (PDB: 5WLC and 6KE6) that included all ribosomal proteins and RNA was used as initial template for rigid body docking into the state H composite map since it is of highest resolution. All template ribosomal protein models were manually adjusted using COOT40. State H was then used as a template to build the proteins and RNA into the other 15 states with manual adjustments in COOT. The final models for the 16 states were real-space refined with three cycles of refinement in PHENIX using phenix.real_space_refine41 using secondary structure restraints for proteins and RNA. In regions with medium to low resolution, protein sidechains were trimmed to the Cβ position after all-atom refinement. The final model refinement statistics can be found in Supplementary Table 1. The maps and models were analysed and visualized in ChimeraX38.
SSU processome predictome
Proteins present within states A–O together with 14 exosome proteins were screened for binary interactions. The resulting 3,570 unique interactions were screened using the default settings in the AlphaPulldown23 implementation of Alphafold-Multimer22. For the SSU processome predictome (Fig. 2a) the median ipTM_pTM score of five models was plotted (Supplementary Table 3).
Yeast growth assays
The rrp6Δ utp14 Δ strain and rrp6Δ utp18 Δ utp14 Δ strain in the BY4741 background were used for all studies. These strains were transformed with two yeast centromeric vectors, one vector under URA3 selection bearing wild-type RRP6 and UTP14 derived from pRSII416 and second vector under LEU2 selection bearing wild-type RRP6 or rrp6 alleles containing mutations or c-terminal truncations in conjunction with UTP14 wild-type or utp14 alleles containing mutations derived from pRSII415. Strains carrying both pRSII415 and pRSII416 plasmids were selected on minimal medium (SD-Ura and Leu) after transformation. Colonies grown on the selection plates were selected and grown in minimal medium (SD-Ura and Leu) liquid cultures. Loss of the URA3 plasmid was done on minimal medium agar plates (SD–Leu + 5-FOA) plates by spotting serial tenfold dilutions (starting at OD at 600 nm of 1) of liquid cultures. Growth was monitored at 30 °C over a period of 5 days.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.