Patient recruitment
A total of 206 participants undergoing nasal surgery were recruited between 2011 and 2024 from the Department of Rhinology and Allergy, Beijing TongRen Hospital (Extended Data Table 2). Among them, 172 were diagnosed with NP and 34 diagnosed with septal deviation (considered to be HCs in the current study). Patients who had immunodeficiency, pregnancy, chronic rhinosinusitis without NPs, allergic fungal sinusitis, cystic fibrosis or had taken oral corticosteroids 4 weeks before the surgery were excluded from the study. See Extended Data Table 1 for patient details. The study was approved by the Medical Ethics Committee of Beijing TongRen Hospital (TREC2009-27, TRECKY2019-027 and TREC2022-KY127). All participants provided written informed consent.
The diagnosis of NP was based on the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2007 guidelines51. The comorbid asthma diagnosis was confirmed by pulmonologists according to the clinical presentation and results from the pulmonary function tests, following the Global Strategy for Asthma 2008 guidelines52. For research purposes, patients with NP were further grouped on the basis of their disease severity, with those placed in the svNP group having disease recurrence or comorbid asthma up to 1 year after surgery, or both.
Patient tissue processing
Polyps tissues from patients with NP and uncinate tissues from patients with septal deviation were collected during endoscopic sinus surgery. One part of the tissue was immediately digested into single-cell suspension for scRNA-seq and flow cytometry analyses. Another part of the tissue was either fixed in the 4% paraformaldehyde for histology or snap-frozen in liquid nitrogen for long-term storage at −80 °C for subsequent enzyme-linked immunosorbent assay (ELISA) and bulk sequencing analyses.
Preparation of single-cell suspension from human nasal tissues
Fresh human nasal tissues were first sliced into small pieces in supplemented Roswell Park Memorial Institute (RPMI) medium (Invitrogen) containing 2% FBS (Invitrogen), 2 mM l-Glutamine (Invitrogen), 50 IU ml−1 penicillin and 50 μg ml−1 streptomycin (Invitrogen). The nasal tissues were then collected by centrifugation and fragmented by the gentleMACS dissociator (Miltenyi Biotec) according to the manufacturer’s instructions. Next, nasal tissues were again collected by centrifugation and further digested with 2 mg ml−1 collagenase (Worthington) and 0.04 mg ml−1 DNases I (Roche) in supplemented RPMI medium for 40 min at 37 °C. The released cells were resuspended and filtered through a 70 μm cell strainer (BD Bioscience). Red blood cells were lysed by using Versalyse (Beckman Coulter).
PBMC isolation
Whole blood was collected during regular follow-up visits, and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using the Biosci human lymphocyte separation medium (Dakewe) following the manufacturer’s instructions.
T cell stimulation
For degranulation assays, lymphocytes were first enriched from human nasal cells by density centrifugation. Briefly, cells were resuspended in 40% Percoll (GE), layered on top of 70% Percoll and centrifuged at 1,500g for 15 min. Subsequently, the cells were stimulated by either plate-bound anti-CD3 (9 μg ml−1, clone OKT3) and anti-CD28 (9 μg ml−1, clone CD28.2) antibodies or PMA (50 ng ml−1) and ionomycin (1 μg ml−1) for 4 h in the presence of the FITC-anti-LAMP1 antibodies (clone H4A3, BioLegend), and monensin (5 μΜ, BD) was added after 1 h.
Mice
C57BL/6J (Jax 664), Rosa26-creERT2 (Jax 8463), CD45.1 (Jax 2014), OT-I (Jax 3831), Cd4-cre (Jax 22071) and Tcrb−/−Tcrd−/− (Jax 2122) mice were originally from the Jackson Laboratory. Gzmkfl/fl mice and Gzmk−/− mice (constructed on C57BL/6J background) were from GemPharmatech and Cyagen, respectively. All animals were maintained in specific pathogen-free facilities at Tsinghua University (THU), with filtered air, sterile pellet food, an acidified watering system and a 12-h light/12-h dark cycle. The temperature was kept at 22–26 °C and humidity at 40–70%. Experiments were performed according to the governmental and institutional guidelines for animal welfare and approved by the Institutional Animal Care and Use Committee at THU.
For each experiment, at least two animals were included in each group, and data were pooled from 2–4 independent experiments. Sex- and age-matched animals of the indicated genotypes were randomly assigned to different groups in each experiment. The mouse lung function assays were run by researchers blinded to group allocations. For other analyses that were based on subjective instrumental measurements, blinding was not performed.
Mouse airway inflammatory disease model construction
Mouse airway inflammatory disease models were built by two approaches. In the first approach, mice were first primed by intraperitoneal immunization with 50 μg of OVA (Sigma-Aldrich) emulsified in 50% alum (Invitrogen) with 1 μg of LPS (Sigma-Aldrich). The mice were then challenged by 5% aerosolized OVA (w/v) in a whole-body exposure chamber (Yiyankeji) for 30 min per day for three consecutive days starting from 1 week postimmunization. The process was repeated one or twice at a 7 day interval, and analyses were performed at the time indicated. For the induction of Gzmk deletion, the Rosa26-creERT2 Gzmk+/+ and Gzmkfl/fl mice were gavaged daily with 2 mg of tamoxifen (ApexBio) dissolved in 200 μl of sunflower seed oil (JSENB) for five consecutive days at the indicated time. In some experiments, Tcrb−/−Tcrd−/− mice were used for asthma induction. These mice were adoptively transferred with 6 × 106 splenic CD4+ T cells and 3 × 106 splenic CD8+ T cells from the Gzmk+/+ or Gzmk−/− mice, purified by magnetic beads (Miltenyi Biotec), 5 days before the intraperitoneal OVA immunization. PPACK (MedChemExpress) and Z-IETD-FMK (Selleck) was prepared in PBS containing 3% DMSO at a concentration of 312.5 μg ml−1 for the inhibition of GZMK and GZMB activity, respectively. Each mouse was injected intraperitoneally with 62.5 μg inhibitor every 2 days from 8 to 18 days postimmunization as indicated.
Alternatively, airway inflammation was in induced in animals adoptively transferred with OT-I T cells. Briefly, B6 mice were primed by intraperitoneal immunization of OVA as described above. Then 1 × 106 OT-I cells were transferred into the primed mice 6 days postimmunization, which were boosted with OVA intraperitoneally the following day. The mice were challenge with OVA inhalation for three consecutive days 1 week later, and the analyses were performed at the indicated time. For experiments involving C3−/− and matched control animals, 0.5 μg of LPS were used in the first OVA immunization and 2 × 106 OT-I cells were transferred.
Cell culture and retroviral transduction
Splenic CD8+ T cells were isolated from OT-I mice by CD8a Microbeads (Miltenyi), and expanded in vitro by plate-bound anti-CD3 and anti-CD28 antibodies (bioXcell) in complete RPMI medium (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, 50 IU ml−1 penicillin, 50 μg ml−1 streptomycin and 10 ng ml−1 recombinant IL-2 (Peprotech). Subsequently, these OT-I cells were transduced with retrovirus encoding only green fluorescent protein (GFP), GFP with either Gzmk or GzmkS213A or GFP with Gzmb. GFP+ OT-I cells were sorted on a AriaIII sorter (BD) and cultured in vitro for two more days before being adoptively transferred into recipient mice.
Bronchoalveolar fluid collection
The mice were anaesthetized by intraperitoneal injection of 1.2% avertin (Sigma-Aldrich) at a dose of 300 mg kg−1. The tracheas were cannulated and lavaged three times, each time with 800 μl of cold PBS. The collected BALF was centrifuged at 300g for 3 min, and the cell pellets were subjected to FACS analyses.
Flow cytometry
Human cells isolated from nasal tissues and peripheral blood were washed once with PBS, blocked by the human TruStain blocking reagents (BioLegend) and stained with different surface antibodies in FACS buffer (PBS with 1% FBS and 5 mM EDTA) for 30 min on ice. For intracellular staining, the cells were fixed and permeabilized by the CytoFix/Perm kit (BD), and stained with antibodies against intracellular antigens for 30 min on ice. Staining reagents include zombie yellow, PerCP-Cy5.5-anti-CD45 (clone HI30), PE-anti-CD8 (clone HIT8a), BV711-anti-CD8 (clone HIT8a), APC-Cy7-anti-NCAM (clone HCD56), PE-cy7-anti-KLRG1 (clone SA231A2); PE-Cy5-anti-KLRC1 (clone S19004C), FITC-anti-ITGAE (clone Ber-ACT8), PE-Dazzle594-anti-ITGAE (clone Ber-ACT8), BV421-anti-CD27 (clone O323), PE-anti-GZMK (clone GM26E7) and APC-anti-GZMB (clone GB11) from BioLegend, APC-H7-anti-CD19 (clone SJ25C1) from BD Biosciences. For mouse models, BALF cells were first blocked with 10 μg ml−1 2.4G2 antibodies (BioXCell), and stained with different antibodies in FACS buffer on ice for 30 min. Staining reagents include AF700-anti-CD11c (clone HL3, BD), eFluor660-anti-CD3 (clone 17A2, eBioscience), APC-anti-I-Ab (clone AF6-120.1, BioLegend), FITC-anti-Ly6G (clone IA8, BD), PE-Cy7-anti-CD11b (clone M1/70, BD), PE-anti-Siglec-F (clone E50-2440, BD), APC-Cy7-anti-CD8 (clone 53-6.7, BioLegend), Percp-Cy5.5-anti-CD4 (clone RM4-5, BD) and zombie yellow (BioLegend). Data were acquired on the Aurora full-spectrum cytometer (Cytek). All FACS data were analysed with the FlowJo software (Treestar).
For single-cell sequencing analyses, 10,000-20,000 cells were sorted into 5 μl of PBS containing 1% FBS in microtubes using an Aria xsIII sorter. For human NP tissue cells, CD45+ cells were sorted as indicated in Extended Data Fig. 3a. Human peripheral CD8+ T cells were first enriched from total PBMCs, and sorted as shown in Extended Data Fig. 3d. Briefly, PBMCs were incubated with anti-human CD8a antibody (clone OKT8, Invitrogen) on ice for 30 min, and antibody-bound cells were isolated by using the streptavidin-coupled microbeads (Miltenyi) following the manufacturer’s instructions. For the analyses of mouse BALF CD8+ T cells, CD45+ cells were sorted with the exclusion of eosinophils and neutrophils as indicated in Extended Data Fig. 5b.
Immunohistochemistry analyses
Nasal tissues were fixed in 4% formaldehyde for 4 h, dehydrated in 30% sucrose at 4 °C overnight and frozen in the Tissue-Tek O.C.T. Compound (Sakura Finetek). Cryo-sections of 8 µm were prepared using a microtome (Leica). The sections were rehydrated in staining buffer (0.05% Tween 20 in 0.1 M Tris-HCl), and blocked with the immunofluorescence blocking buffer (CST) for 30 min. After staining with different antibodies in staining buffer at 4 °C overnight, the sections were incubated with 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI, 1 μg ml−1, Sigma-Aldrich) for 10 min as indicated, and mounted with the fluoromount aqueous mounting medium (Sigma-Aldrich). Images of intact tissue sections were acquired using the Zeiss Axio Scan Z1 slide scanner (Zeiss); alternatively, zoom images of the GZMK-expressing T cell aggregates were acquired using the LSM 710 confocal microscope (Zeiss). Staining reagents include PE-anti-GZMK (clone GM26E7, BioLegend), APC-anti-CD8 (clone HIT8a, BD), FITC-anti-KLRG1 (clone 2F1/KLRG1, BioLegend), APC-anti-CD4 (clone OKT4, BioLegend), FITC-anti-PTGDR2 (clone BM16, BioLegend) and FITC-anti-complement C3b/iC3b (3E7/C3b, BioLegend).
Areas of GZMK-expressing T cell aggregation from whole tissue scanned images were quantified independently by investigators blinded to the patient information using ZEN (Zeiss). The numbers of different cell subsets were quantified from confocal images by Imaris (Bitplane) on the basis of expression of different marker genes.
Histologic evaluation of NP immune cell subsets
Fixed NP tissues were embedded in paraffin and stained with haematoxylin and eosin. The absolute numbers of eosinophils were quantified in three randomly selected high-power magnification fields (×400) by two pathologists.
Bulk TCR sequencing
Total RNAs were extracted from the nasal tissues by using the HiPure Total RNA Mini Kit (Magen). Reverse transcription of 10–500 ng input RNA was performed following the standard Smart-seq2 protocol53, with the following modifications: The polyT reverse transcription primer was replaced by the T30VN oligo (5′ TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN-3′) and the original template switch oligo (TSO) was replaced by a 26N unique molecular identifier (UMI)-containing TSO (5′-CTACACGACGCTCTTCCGATCTNNNNNNNNNNNNNNNNNNNNNNNNNNTTTCTTATATrGrGrG-3′, rG represents riboguanosines). TSO was removed by addition of 1 μl of ExoI (NEB) to each reverse transcription reaction and incubation at 37 °C for 15 min, which was subsequently inactivated by incubation at 85 °C for 5 min. TCR enrichment was done in two steps. The reverse transcription product was first mixed with 25 μl of 2× KAPA HiFi HotStart ReadyMix (KAPA Biosystems), 10 μM B/T enrich F1 primer (5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTC-3′; Sangon Biotech), 50 μM of each TCR outer primers (TCRα, 5′-TGAAGGCGTTTGCACATGCA-3′; TCRβ, 5′-TCAGGCAGTATCTGGAGTCATTGAG-3′; Sangon Biotech) to a total volume of 50 μl, and amplification was done with the following program (98 °C 45 s, 11 cycles of 98 °C 20 s, 67 °C 30 s, 72 °C 1 min; 72 °C 1 min). The PCR products was then purified by VAHTS DNA Clean Beads (Vazyme) at a 0.8 to 1 (beads to sample) ratio according to the manufacturer’s instructions. Afterwards, the second step of TCR enrichment was performed by mixing the purified products with 25 μl of 2× KAPA HiFi HotStart ReadyMix, 10 μM B/T enrich F2 primer (5′-AATGATACGGCGACCACCGA-3′; Sangon Biotec), 50 μM of each TCR inner primers (TCRα, 5′-AGTCTCTCAGCTGGTACACG-3′; TCRβ, 5′-TCTGATGGCTCAAACACAGC-3′; Sangon Biotec) to a total volume of 50 μl and amplified by the following program (98 °C 45 s, eight cycles of 98 °C 20 s, 67 °C 30 s, 72 °C 1 min; 72 °C 1 min). The products were put through a double-sided size selection by the VAHTS DNA Clean Beads, at 0.5 to 1 and 0.3 to 1 (bead to sample) ratios, respectively. Subsequently, 1 ng of the purified products was tagmented by Tn5 transposase (Vazyme) in 20 μl of TD reaction buffer (10 mM Tris-Cl pH 7.6, 5 mM MgCl2, 10% DMF) at 55 °C for 10 min. The reaction was mixed with 2 μl of 0.2% SDS and incubated at 70 °C for 20 min for transposase inactivation. Index PCR was performed by mixing the tagmentation product with 3 μl of 10 μM unique dual index primers and 25 μl of Q5 High-Fidelity 2× Master Mix (NEB), and amplified with the following program (72 °C 3 min, 98 °C 30 s, 12 cycles of 98 °C 15 s, 60 °C 30 s, 72 °C 3 min; 72 °C 5 min). The PCR products were then purified with VAHTS DNA Clean Beads at a ratio of 0.8 to 1 (bead to sample). The constructed libraries were sequenced on Novaseq 6000 System (Illumina) in a 2× 150 paired-end mode.
scRNA and scTCR sequencing
For human tissue CD45+ cells and tissue CD8+ T cell subsets, the scRNA and scTCR libraries were prepared using the Chromium Single Cell V(D)J Reagent Kits (10X Genomics). And the Chromium Next GEM Single Cell 5′ Kit v2 (10X Genomics) were used to construct libraries for human peripheral blood samples and mouse BALF cells. The library sequencing was performed on an NovaSeq 6000 System.
Laser-captured microdissection and bulk RNA sequencing analyses
RNA sequencing of the GZMK+ cell aggregated tissue areas was performed following the Geo-seq protocol54. Briefly, NP tissues were embedded in the Tissue-Tek O.C.T. compound, snap-frozen by liquid nitrogen and sectioned into 10 μm consecutive slices. The GZMK+ cell aggregated areas were first confirmed by immunohistochemistry staining with DAPI and PE-anti-GZMK antibodies, then an adjacent slice was attached to the PEN MembraneSlide (catalogue no. 11505151, Leica) and stained with crystal violet. The matched GZMK+ cell aggregated and control tissue areas were excised and added to 50 μl of guanidine thiocyanate, and incubated at 42 °C for 15 min. The RNA was then precipitated by mixing the samples with 150 μl of deionized H2O, 600 μl of anhydrous ethanol, 20 μl of 1.5 M sodium acetate (pH 6.5) and 1 μl of glycogen (20 mg ml−1). The mixtures were placed at −80 °C for 30 min and centrifuged at 12,000g for 30 min at 4 °C. After washed by 75% (v/v) ethanol, the precipitated RNAs were resuspended in H2O for library construction using the Smart-seq2 protocol53.
The sequencing data were mapped and quantified by Salmon55. Samples with mapping rates lower than 20% was excluded from the analyses. Differential expression analyses were performed by DESeq2 (ref. 56), and principal component analyses was done by using the ‘plotPCA’ function after variance-stabilizing transformation.
scRNA-seq analyses
CellRanger (v.3.0) was used to generate gene expression matrix for each cell, which was further processed by Seurat (v.3.0.2)57 for data combination, dimension reduction, clustering and gene differential expression analysis. For human tissue CD45+ cells, data from different samples were first pooled by the ‘merge’ function. Subsets containing αβ T cells and myeloid cells were identified, and batch effects were removed by the ‘IntegrateData’ function. For the analyses of human tissue CD8+ T cell subsets, data from different samples were first pooled by the ‘merge’ function, then the ‘FindTransferAnchors’, ‘TransferData’, ‘MapQuery’ and ‘IntegrateEmbeddings’ functions in Seurat v.4.3.0.1 were used for cluster prediction and projection of the query onto the reference uniform manifold approximation and projection (UMAP) structure on the basis of the total tissue αβ T cell dataset. For transcriptome association analyses in Fig. 2g, we first calculated the average gene expression in human tissue αβ T cell and blood CD8+ T cell clusters, and ranked the genes by their standard deviations across different clusters. Then the top 2,000 genes were used to form the average expression matrix, which was used to calculate the Spearman’s rank correlation coefficient. For the comparison between T1 and B2 cells described in Extended Data Figs. 5 and 6, we first pooled the T1 and blood CD8+ T cells from NP11 and NP13 by the ‘merge’ function in Seurat. The average UMI for each gene in the two subsets were then graphed and the Spearman correlation coefficient was calculated. For trajectory analyses, clones shared between tissue and blood were subsampled and used for UMAP dimensional reduction, and the pseudotime for these cells was calculated by using Monocle3 (ref. 58) (v.1.3.1). For cell–cell interaction analyses described in Supplementary Fig. 8, each indicated immune cell subset was downsampled to ≤100 cells for downstream analyses by using the ‘subset’ function in Seurat. The gene expression matrix was input into CellphoneDB30 (v.3) to calculate the receptor-ligand interactions. Network diagrams were graphed by Cytoscape59 (v.3.10.1) and bubble plots were graphed by gglot2 package.
For mouse BALF cells, we integrated data from two biological replicates with the ‘IntegrateData’ function and performed UMAP dimension reduction as well as clustering. Then the CD8+ T cells were put through another round of dimension reduction and clustering for further analyses.
TCR repertoire analyses
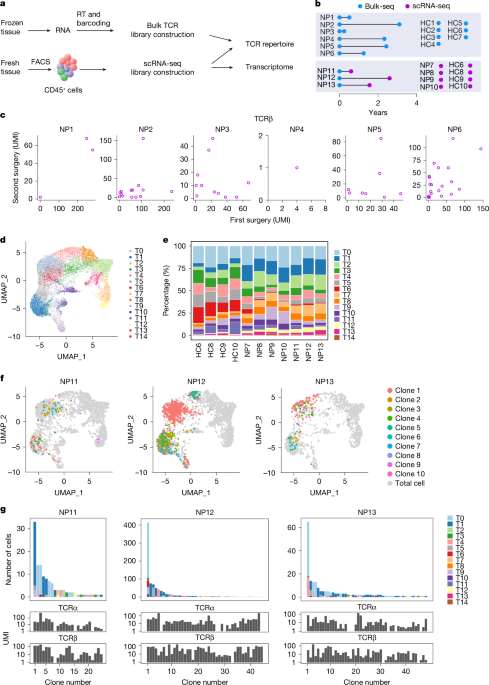
For the bulk TCR-seq dataset, adaptors were first removed by Cutadapt60, then the TCR sequences were assembled by TRUST4 (ref. 61), which identified the first N16 of N26 within the TSO oligos as UMI and resulted in filtered contig files containing high-quality assembled TCR contigs for each UMI. UMIs with more than one TCR contigs were excluded from the analyses. For the scTCR-seq datasets, CellRanger was used to assemble TCRs for single cells. TCR contigs were annotated using Igblast62 (v.1.16.0), and only productive TCR contigs with complete VDJ regions were used for downstream analysis. In addition, cells with many productive TCRα or TCRβ contigs were removed from the scTCR dataset. Common TCR clones between different datasets were identified by the shared CDR3 nucleic acid sequences and common V(D)J germline usage. In the case of scTCR-seq data, both α and β chains were considered. The circus plots were graphed by using the circlize package, and the rest of the graphs were visualized using ggplot2 and Seurat.
For TCR specificity prediction, 37,483 αβ TCR pairs with annotated cognate antigens were collected from VDJdb database13 and the Immune Epitope Database14 and integrated with 1,311 T1 TCRs from our own scTCR-seq dataset. The resulted TCR list was analysed using the GLIPH2 (ref. 15) web portal (http://50.255.35.37:8080). TCRs were clustered on the basis of both local and global similarity, and the clusters were filtered by the following criteria: Fisher_score ≤0.05 and vb_score ≤0.05. All clusters containing T1 cell TCRs were graphed using Cytoscape (v.3.10.1).
ELISA and Luminex assay
Nasal tissues were weighed and homogenized mechanically using the TissueLyser LT bead mill (Qiagen). RIPA buffer containing the complete protease inhibitor cocktail (Roche) was added to the homogenates (10 ml g−1). The lysates were frozen and thawed twice, centrifuged at 12,000g for 5 min at 4 °C and the supernatants were used for GZMK measurements by ELISA (Reddot Biotech) and IL-5 measurements by Luminex xMAP assay (Thermo Fisher) on a Bio-Plex 200 system (BioRad). All analyses were done following the manufacturer’s instructions.
Lung function assay
Airway responsiveness of mice was assessed using the FlexiVent system (SCIREQ) following the manufacturer’s instructions. Tracheotomy and endotracheal intubation were performed after anaesthetizing the mice with 80 mg kg−1 pentobarbital sodium (Sigma-Aldrich). Airways resistance and compliance were measured on sequential exposure to increasing doses of methacholine (Sigma-Aldrich) dissolved in sterile normal saline (0, 6, 12, 24 and 48 mg ml−1).
AB-PAS staining
The left mouse lungs were fixed in 4% formaldehyde for 4 h, dehydrated in 30% sucrose for 2 days at room temperature and frozen in Tissue-Tek O.C.T. Compound at −80 °C. The tissue blocks were cryo-sectioned into 10 μm slices, and Alcian Blue-Periodic acid Schiff (AB-PAS) staining was performed using the AB-PAS solution set (Servicebio) according to the manufacturer’s instructions. The panoramic images of a whole lung section were acquired for each animal using the Zeiss Axio Scan Z1 slide scanner, and zoom-in views were acquired by a Nikon eclipse Ts2R microscope (Nikon). PAS+ areas in each airway were quantified using ImageJ (NIH) with the same threshold and normalized to the perimeter of the airway. The mean value was calculated from the four airways with the highest normalized PAS+ area for each section, and assigned to the corresponding animal.
Quantitative PCR
RNA was extracted from sorted mouse cells by trizol and reverse transcribed into complementary DNA (cDNA) by 5× All-In-One MasterMix (abm). Quantitative PCR were performed with Blastaq 2× quantitative PCR Mix (abm) on the CFX Connect Real-Time System (BioRad). Primers used were listed as follows: Actb-F 5′-CCTAAGAGGAGGATGGTCGC, Actb-R 5′-CTCAAGTCAGTGTACAGGCCA; Gzmk-F 5′-TGTCCAACTGCTTCACCTGGG and Gzmk-R 5′-GCCACCAGAGTCACCCTTGCA. Gzmk level was normalized to the level Actb.
Expression and purification of recombinant human GZMK
Recombinant human GZMK were produced and purified as described previously63. Briefly, the cDNA for human GZMK was cloned into the pHL vector. PCR mutagenesis was performed to produce the enzymatic inactive GZMK-S214A mutant. 293-F cells (obtained from Thermo Fisher; validated by the provider; mycoplasma negative) were seeded in 293-TII medium (Sino Biological) at a concentration of 2 × 106 cells per ml one night before the transfection. Subsequently, the plasmids were mixed with linear polyethylenimine (molecular weight 40,000, Yeasen Biotechnology) at a ratio of 1:3, and transfected into 293-F cells. The supernatants were harvested after 3 days, and the granzymes containing a C-terminal His-tag were captured onto a nickel affinity column (Yeasen Biotechnology). The eluted proteins (containing an engineered enterokinase site at the N terminus) were digested by the enterokinase (Beyotime Biotechnology), and cation exchange chromatography was carried out using the Resource S column (Cytiva) on the AKTA Purifier 10 system (GE) to further purify the protein.
Protease activity assay of recombinant GZMK
The protease activity of GZMK was measured by a FRET-based assay (Extended Data Fig. 4b) using a synthetic fluorogenic peptide substrate, DABCYL-GDGRSIMTE-EDANS (Sangon Biotechnology). The reactions were performed in 20 mM HEPES, pH 7.0 at 37 °C with 0.5 µM protease and 7.5 µM peptide substrate. After 15 min, the fluorescence was monitored at 490 nm with an excitation wavelength of 340 nm using Varioskan Flash reader (Thermo Fisher) in a 96-well microplate. Trypsin (Promega) was used as a positive control, and lysozyme (Sigma-Aldrich) was used as a negative control.
GZMK pull-down assay
Purified GZMK-S214A was first biotinylated by reacting with the EZ-Link NHS-LC-Biotin (Thermo Fisher) according to the manufacturer’s instructions, and coupled to the M-280 Streptavidin Dynabeads (Thermo Fisher). To do that, 200 µl of Dynabeads were equilibrated in PBS and incubated with 20 µg biotinylated GZMK-S214A for 30 min at 4 °C. NP lysates were prepared as described except with a different lysis buffer (20 mM Tris, 150 mM NaCl, 1% Triton X-100) supplemented with protease and phosphatase inhibitors (Beyotime Biotechnology). Subsequently, 300 µl of the lysates from each sample were precleared by 200 µl of Dynabeads at 4 °C for 4 h, then the precleared lysates were incubated with the biotinylated GZMK-S214A-Dynabeads overnight at 4 °C. The Dynabeads were washed with the lysis buffer, and the associated proteins were eluted with 0.1 M Glycine (pH 2.5) and neutralized with 1 M Tris (pH 7.5).
Mass spectrometry and data analysis
Proteins pulled down by GZMK-S214A-Dynabeads or Dynabeads only were resolved by 4–20% SDS–PAGE and visualized by Coomassie brilliant blue staining. The protein bands were excised and subjected to in-gel digestion. Gel pieces were washed in 50 mM NH4HCO3 (pH 8) and destained in 50% 50 mM NH4HCO3 in 50% ACN. The proteins were then incubated with 10 mM DTT (Sigma-Aldrich) for 60 min at 60 °C followed by 20 mM IAA (Sigma-Aldrich) for 30 min in the dark at room temperature. Trypsin (Promega) was added at 1:50 ratio (w/w), and the mixture was incubated overnight at 37 °C. Finally, the samples were cleaned on a C18 cartridge (Waters Corporation) and ready for LC–MS analyses. LC–MS was carried out using an UltiMate 3000 UHPLC System (Thermo Fisher) connected to a Fusion Lumos Tribrid mass spectrometer (Thermo Fisher). Mass spectrometry data were analysed using the SEQUEST HT search engine against a UniProt Swiss-Prot database with Proteome Discoverer v.2.3 (Thermo Fisher). Among the identified GZMK-interacting proteins, 56 appeared in all samples analysed. These proteins were ranked by SEQUEST scores and the abundance ratio (samplepeptide counts/controlpeptide counts). The protein–protein interaction analysis of GZMK-interacting proteins was done with Cytoscape59 (v.3.9.1).
Edman sequencing
The phenylthiohydantoin amino acid was separated in the reversed-phase mode of high-performance liquid chromatography using the differences between the retention times of different amino acids, and the amount of UV (ultraviolet light) absorbance at specific wavelengths was detected. The samples were transferred to the polyvinyl difluoride membrane and five cycles were set. The amino acid sequences of each sample were determined from the chromatograms obtained in each cycle evaluation performed by comparing chromatograms with those in the previous and subsequent cycles and identifying the phenylthiohydantoin amino acids that had the greatest increase in abundance.
GZMK cleavage assay
The recombinant SET protein (Solarbio) and serum-purified complement C2, C3, C4 and C5 (Complement Technology) at a concentration of 120 μg ml−1 were incubated with 24 μg ml−1 recombinant GZMK in PBS at 37 °C for the indicated period of time. To test the cleavage of DMBT1, NP tissue lysates were incubated with increasing dose of recombinant GZMK at 37 °C for 1 h. The reactions were stopped by the addition of 6× SDS loading buffer (reducing, TransGen), and the samples were then boiled for 15 min and resolved by SDS–PAGE. Serum-purified C3a, C3b, C2a, C4b (Complement Technology) and recombinant GZMK were separately loaded as controls. Target proteins were either visualized by Coomassie brilliant blue staining or detected by immunoblotting with different antibodies. The antibodies used include anti-SET (clone EPR12973, Abcam), anti-GZMK (clone EPR24601-164, Abcam), anti-C2 (clone EPR17979, Abcam), anti-C3 (clone EPR19394, Abcam), anti-C3a/C3a-desArg (clone 2991, Hycultbiotech), anti-C4α (clone C-2, Santa Cruz), anti-C5a/C5a-desArg (clone C17/5, Abcam), anti-DMBT1 (clone G-4, Santa Cruz), anti-ACTB (clone C4, Santa Cruz), HRP-Goat-anti-Mouse IgG (H+L) (Beyotime Biotechnology) and HRP-Goat-anti-Rabbit IgG (H+L) (Beyotime Biotechnology). Immunoblots were developed by the Super ECL Detection Reagent (Yeasen Biotechnology).
For the comparison between GZMK and different C3 convertases, serum-purified human C3 (Complement Technology, 1.75 µM) were incubated with GZMK (1 µM) or other C3 convertases (C3bBb or C4b2a; 1 µM) for 20 min at 37 °C. C3bBb was formed by mixing C3b (Complement Technology, 2 µM), FB (Complement Technology, 1 µM) and FD (Complement Technology, 500 nM) in HBS-Mg buffer (20 mM HEPES, 140 mM NaCl, 5 mM MgCl2) for 2 min at 37 °C and the reaction was terminated by 5 mM EDTA. C4b2a was formed by incubation of C4b (Complement Technology, 1 µM), C1s (Complement Technology, 0.58 µM) and C2 (Complement Technology, 1 µM) in PBS with 0.5 mM CaCl2, 2 mM MgCI2 and 40 mM NaCl for 5 min at 37 °C, and the reaction was terminated by 2 mM EDTA. The amount of C3a shown on the Coomassie brilliant blue-stained gels were quantified by using ImageJ, and the data were normalized to the C4b2a group.
Purification of C3a and measurement of C3a activity
C3a converted by different C3 convertases were purified by size-exclusion chromatography (SEC). Specifically, the cleavage products were separated by an SEC column (Acclaim SEC-1000, 4.6 × 300 mm, Thermo Fisher) on a Vanquish HPLC system (Thermo Fisher) with the UV detector set to detect absorbance at 280 nm. PBS buffer (pH 7.4) was used as the mobile phase at a 0.25 ml min−1 flow rate for elution. SEC fractions were collected automatically and further concentrated by the Amicon ultra centrifugal unit (3 kDa molecular weight cutoff, Millipore). The purity of C3a was confirmed by SDS–PAGE (4–20%) and Coomassie brilliant blue staining.
THP1 cells (obtained from the National Infrastructure of Cell Line Resource, China; validated by the provider; mycoplasma negative) were maintained in RPMI-1640 medium (10% FBS, 1% penicillin and 1% streptomycin) and used to measure C3a activities. Briefly, the cells were serum-starved overnight, incubated with 5 nM C3a from the indicated sources at 37 °C for 5 min, and lysed with RIPA buffer containing protease inhibitors (Roche) and phosphatase inhibitors (Thermo Scientific). Immunoblotting was performed using anti-phospho-Erk1/2 (Cell Signaling Technology) and anti-Erk1/2 (clone 137F5, Cell Signaling Technology) antibodies. Data were quantified by ImageJ and normalized to the untreated control.
Haemolytic assay
Serum-purified human C5b6 (600 pM) was first incubated with recombinant GZMK or Trypsin (600 pM) at 37 °C for the indicated time. The reaction mix was then added to chicken erythrocytes (3.3 × 107 per ml, Sbjbio) suspended in the Veronal Buffered Saline (pH 7.4), and incubated at 37 °C for 5 min. Subsequently, serum-purified C7 was added (15 nM) to the erythrocytes and the mixture was incubated at 37 °C for 15 min. Finally, a mix of serum-purified C8 (10 nM) and C9 (25 nM) were add to the cells, and further incubated at 37 °C for 30 min. The remaining intact erythrocytes were removed by centrifugation at 1,000g 4 °C for 2 min, and haemolysis was determined by absorbance measurement at 405 nm.
Statistical analyses
Statistical analyses were performed by Prism v.9 (GraphPad). Mann–Whitney U-tests were used to calculate P values from the human data, and two-sided Student’s t-tests were used for mouse data analyses. Categorical variables were analysed using the Chi-square test. Receiver-operating characteristics analysis was also performed in R (v.4.3.3) with the pROC package for prediction of polyp recurrence or comorbid asthma. The multiple linear regression analyses were performed using the emmeans package in R (v.4.3.3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.