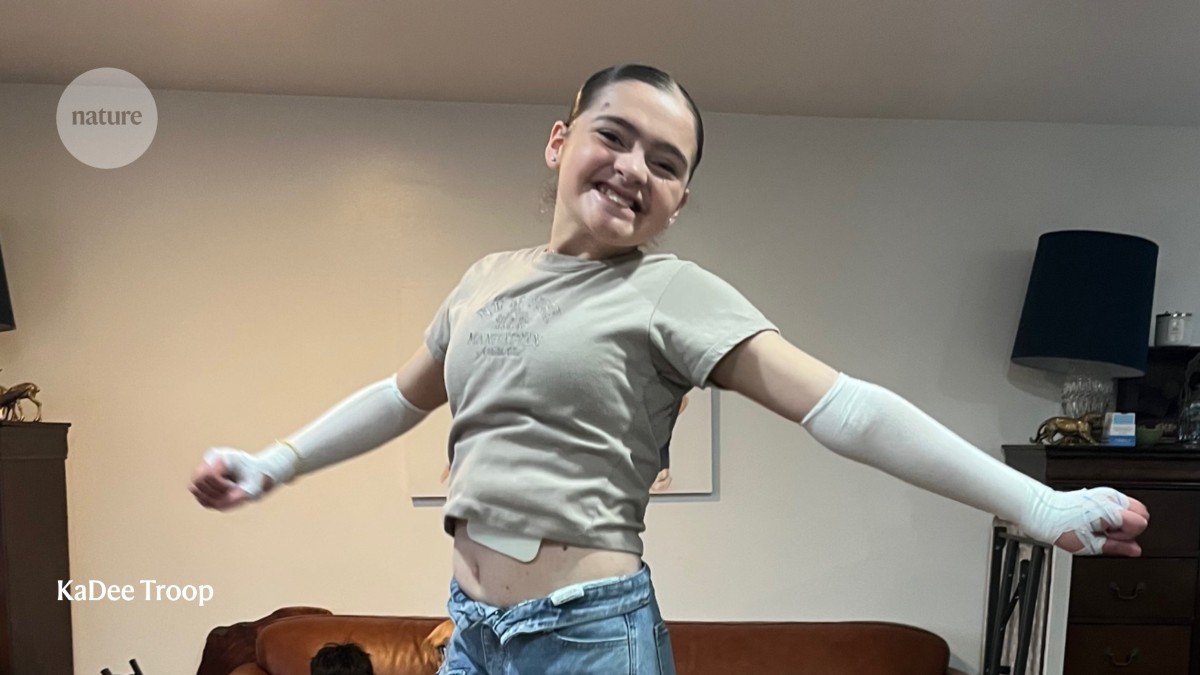
A gel-based gene therapy has helped Jayne Troop to lead an active life. Credit: KaDee Troop
KaDee Troop is the mother of seven adopted children, four of whom have a rare genetic disorder that causes their skin to blister and tear at the slightest touch. Wounds heal slowly — if at all — and with each re-injury, the skin becomes thinner and more fragile. For most of the children’s lives, hope came in the form of gauze and bandages. That is, until 2023, when it arrived in a bottle.
That year, US regulators approved the first gene therapy for a form of epidermolysis bullosa, the condition that affects the Troop children and is sometimes referred to as butterfly skin. All four children began applying the gel-based treatment to their raw, vulnerable lesions; the results were profound. Wounds that had stubbornly resisted healing for years began to close.
With the gene-therapy safety net, one of the children, Jayne, now 14, feels confident enough to perform backflips on the trampoline; Alex, at 12, barrels down rocky trails on his mountain bike; Ellen, 13, has gained independence, doing wound care by herself; and Zack, 22, lifts weights using a custom arm prosthetic with dumbbell-clamping grips and a shoulder harness fitted over his skin that was once unable to bear the strain. “Things have changed in a big way,” says KaDee, who lives with her family in Bountiful, Utah.
Nature Outlook: Skin
It was a turning point for the Troops, and for the field of genetic medicine. The treatment, beremagene geperpavec (B-VEC), became the first gene-replacement therapy approved for a non-cancerous skin disorder, and the first topical gene therapy for at-home use for any disease.
What’s more, the success of B-VEC — developed by Krystal Biotech in Pittsburgh, Pennsylvania — proved that gene therapy could work on the skin’s surface. And it has paved the way for other therapeutic strategies, including the first gene therapy delivered through skin grafts, which was approved this year for a form of the condition known as recessive dystrophic epidermolysis bullosa (RDEB). It offers a complementary approach for addressing inherited skin disorders at their root cause.
These two medicines mark the beginning of a new era in precision treatments for rare genetic skin diseases. They have shifted the focus from symptom management to molecular repair, and given families such as the Troops reason to think that better days are possible.
“None of these treatments we’re talking about are cures,” cautions Emily Gorell, a dermatologist at the University of Colorado Anschutz Medical Campus in Aurora. They target the skin manifestations of these diseases, not internal tissues such as the mucosal linings of the mouth and gastrointestinal tract, which can also be damaged. Cost and access to treatment remain major hurdles, too. Still, Gorell emphasizes, they are a major step forward for a group of disorders that have long been defined by chronic pain and therapeutic neglect. “I’m just hoping for better treatments,” she says, and these offer a meaningful start.
Layers of progress
Work on B-VEC began about 10 years ago when married couple Suma and Krish Krishnan founded Krystal, with an eye to harnessing engineered herpes simplex virus (HSV) for genetic skin diseases.
HSV is best known for causing cold sores. But, as a treatment for melanoma that was approved in 2015 showed, the virus can be repurposed to deliver therapeutic payloads. What’s more, it does so without provoking strong immune reactions — meaning that it can be safely administered many times to sustain or boost its effects.
That made HSV the “obvious choice” to build a therapy that could be applied repeatedly to fragile skin, says Suma Krishnan, who leads research and development efforts at Krystal.
To create B-VEC, Krishnan and her team engineered a non-replicating form of the virus to carry the genetic instructions for making COL7A1, a collagen protein that people with RDEB lack. The treatment, now marketed as Vyjuvek, proved safe and provided lasting wound repair in a clinical trial of 31 individuals, all of whom received standard wound care as well as either the gene therapy or a placebo on size-matched wounds.
After six months, two-thirds of the wounds treated with B-VEC had healed completely, compared with fewer than one-quarter of those treated with the dummy gel1 — a benefit that, in some cases, persisted for two years or more, according to follow-up data reported earlier this year2.
Although B-VEC is the first gene therapy for a skin disease to receive regulatory approval, it is not the first to demonstrate the power of genetic repair in the clinic. As far back as 2006, clinicians in Italy used genetically corrected skin cells to treat a 36-year-old man with junctional epidermolysis bullosa3, a less common and often deadly form of the disease.
The team, co-led by Michele De Luca, a regenerative-medicine specialist at the University of Modena and Reggio Emilia in Italy, took skin samples from the palms of the man’s hands, used a virus to insert a healthy copy of the faulty LAMB3 gene (essential for anchoring layers of skin) and grew sheets of corrected epidermis in the laboratory. The researchers then grafted sticky-note-sized patches of the engineered skin back onto the man’s upper legs. The grafts took hold and regenerated a stable, blister-free covering over areas that had previously been prone to infections and chronic wounds3.
This pioneering approach was repeated years later in other people with junctional disease, including in a seven-year-old in Germany called Hassan, who had lost nearly all of his skin to the disorder4. A decade on, the now-teenager is thriving, his skin is intact and replenishing regularly thanks to a pool of self-renewing stem cells included in the original grafts engineered to carry the genetic correction. “He didn’t develop a single blister,” says De Luca. “It’s amazing.”
Buoyed by such proof-of-concept victories, De Luca and his colleagues had been preparing to launch larger trials of the gene-corrected skin grafts, for both junctional and dystrophic forms of epidermolysis bullosa. But a few years back, the majority shareholder in the company behind the therapy withdrew its commercial funding, and a public bail out by Italian health authorities has so far failed to restart the effort.
Reflecting on this experience, De Luca and his colleagues warned in 2022 that even effective therapies for rare skin diseases were at risk of vanishing — not because they fail in clinical trials, but because they don’t succeed in the marketplace5. “Ultimately,” they wrote, “the patients are those who are damaged the most.”
Skin in the game
Such commercial headwinds didn’t deter Abeona Therapeutics in Cleveland, Ohio, from advancing a similar skin-graft-based product, called prademagene zamikeracel, or pz-cel. Like B-VEC, this gene therapy delivers a working copy of the defective COL7A1 gene that underpins RDEB.
The therapy originated at the Stanford University School of Medicine in California, at which a team led by dermatologists Peter Marinkovich and Jean Tang grafted sheets of gene-corrected skin cells onto chronic wounds and found that most treated areas healed and stayed that way for five years or more, with reductions in pain and itchiness to boot6. A randomized trial sponsored by Abeona later confirmed the therapy’s wound-healing and pain-relief advantages. Many of the participants opted for a second procedure to treat their control wounds — nearby lesions that had received only daily bandaging and other supportive care7. “That always feels so good, knowing that patients are choosing to do it again,” says Tang. “Because it’s not a simple procedure.”
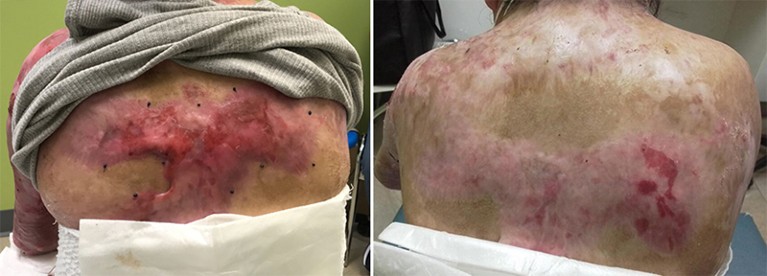
Images of an individual with epidermolysis bullosa taken at the start of a gene-therapy treatment (left) called B-VEC and after 34 months (right). Credit: REF. 2
The US Food and Drug Administration approved pz-cel (marketed as Zevaskyn) in April 2025. Now, Abeona is turning its attention to refining the shape and size of its grafts to better support post-surgical repair of mitten hand — a common complication of RDEB in which scar tissue fuses fingers together, and experienced by Zack Troop. Addressing these contractures is one of the most pressing needs for individuals, according to Abeona’s chief executive Vishwas Seshadri. “That’s what they’re asking for,” he says.
Krystal, for its part, is looking to expand the use of its topical gene delivery beyond skin, starting with efforts to address eye complications that can threaten vision in people with RDEB. In 2021, clinicians at the University of Miami Miller School of Medicine in Florida administered B-VEC as eye drops to a 13-year-old with RDEB who had previously used the gel on his skin. Just as the therapy had helped to heal his skin wounds, it also cleared long-standing blisters and scarring on the surface of his eye8. A more formal trial evaluating this use of the therapy kicked off earlier this year.
In the space of two years, clinicians treating RDEB have gone from having very little to offer to having two gene therapies on hand. Now, they face a new challenge: working out which option offers the greater benefit. “Part of it comes down to what kinds of wounds you’re healing,” says Gorell, who was involved in trials of both approved products.
For recurrent lesions that repeatedly open and close owing to friction or minor trauma, she says, B-VEC’s topical gel might be the more practical option, especially given its ease of use at home. But for deep, non-healing wounds that have resisted other treatments, a graft-based therapy such as pz-cel could provide more durable repair — a benefit that might justify the requirement for a surgical procedure.
Another gene therapy for RDEB might soon join the mix, too. Castle Creek Biosciences in Exton, Pennsylvania, has been advancing an approach that injects millions of gene-corrected skin cells into open wounds. Like pz-cel, the therapy is administered under anaesthesia, but it sidesteps the surgical complexity of graft-based methods. What’s more, unlike pz-cel, Castle Creek’s genetically repaired cells can be cryopreserved, allowing for flexible, repeat dosing — and possibly even injection into intact skin to ward off future wounds.
“There’s a potential for preventative therapy of areas like the hands that may get a lot of trauma,” says Marinkovich, who, in early testing of six individuals with RDEB, found that most wounds treated with the Castle Creek therapy healed almost completely in a few months. (Marinkovich led trials for B-VEC and pz-cel, as well.)
There’s also potential for gene-therapy strategies to be adapted for other inherited skin diseases, such as ichthyosis, that result from single-gene mutations and cause debilitating, life-limiting skin damage. Krystal, for example, is adapting its HSV platform to treat the most common cause of lamellar ichthyosis, a scaly skin disorder triggered by mutations in the TGM1 gene.
In early testing, the Krystal therapy helped to restore functional protein expression in the skin and reduced visible scaling. But ichthyosis affects nearly all of a person’s skin, and treating such an extensive surface area poses challenges around price and safety, says Amy Paller, a paediatric dermatologist at the Northwestern Feinberg School of Medicine in Chicago, Illinois, who led the clinical testing.
She therefore sees greater potential for topical gene therapies in diseases with more localized skin involvement — conditions such as Darier disease, marked by wart-like blemishes in oily areas of the body, or Hailey–Hailey disease, a blistering disorder that affects skin folds around the neck. Preclinical data presented at the Society for Investigative Dermatology annual meeting in May suggest that the strategy is effective in both cultured cells and mouse skin models for each condition.
Towards a lasting fix
As gene therapies make inroads into an increasing number of inherited skin disorders, they could deliver more than just relief from discomfort — they could also prolong lives.
Consider the long-term stakes in RDEB. Up to now, few individuals with the condition have survived past the age of 40. The leading cause of death is a fast-spreading, difficult-to-treat skin cancer called squamous-cell carcinoma, which typically arises in areas of chronic damage and inflammation. By healing skin earlier and more consistently with gene-based therapies, researchers hope to not only improve quality of life, but also help people to live longer.
Still, even the most advanced topical, injected or graft-based treatments stop short of a permanent fix. They restore missing proteins in treated tissues, and some even leave behind gene-corrected stem cells, but these benefits remain confined to the treated areas, without offering much relief for internal symptoms or systemic disease. “Direct in vivo approaches to the skin would be the ultimate answer if efficiency of correcting long-lived skin stem populations can be achieved,” says Waseem Qasim, a gene-therapy specialist at Great Ormond Street Hospital in London.
But without the delivery tools to achieve that kind of deep, durable correction, researchers at Stanford have pursued a workaround. Last year, they described a platform that combines the limitless potential of induced pluripotent stem (iPS) cell technology with the precision of CRISPR gene editing to create tissue replacements for people with epidermolysis bullosa9.
More from Nature Outlooks
“The advantage of the iPS cell technology is you can scale it,” dermatologist and co-author Anthony Oro says. Skin grafts generated in this way could theoretically “cover the whole body multiple times over”, he says. And it should be possible to grow gene-corrected tissue composites for transplanting onto other mucosal surfaces as well, such as the mouth and oesophagus. “This is a programmable tissue regeneration platform,” he says. Working in animal models, the researchers have so far tested four types of engineered graft — each derived from different patient-specific iPS cells. In unpublished work, they have also successfully delivered their cells onto mouse skin using a device that sprays the cells inside a spider-web-like matrix of nanofibres designed to promote long-term healing. Oro says that his team is in discussions with regulators about launching a human trial. “It’s getting close to the clinic,” he says.
Leaving stem-cell reprogramming aside, others in the field are gravitating toward genome-editing technologies, hoping to achieve more targeted genetic correction than is possible with viral gene addition. “It’s going to be cheaper, more versatile and more precise,” says Hilary Sheppard, a molecular biologist at the University of Auckland in New Zealand who has developed gene-editing strategies that address different forms of epidermolysis bullosa.
Unlike pz-cel and other gene therapies that use a virus to randomly insert healthy copies of a gene and leave existing broken ones untouched, gene-editing tools such as CRISPR help scientists to precisely repair mutations in a person’s DNA. This approach ensures more controlled and physiologically appropriate levels of gene expression, with potentially fewer off-target effects than result from conventional gene-replacement strategies.
Gene therapy for skin diseases still faces formidable obstacles, from manufacturing scale-up to access and affordability. The science is complicated. The economics are tough. But B-VEC and pz-cel have proved that fragile skin can be strengthened, and that rare disorders need not be ignored. They offer a glimmer of what’s possible when science meets sustained commitment, and when the surface of the body becomes a platform for deeper biological repair.
For families such as the Troops, the promise of gene therapy is no longer an abstract idea. It is visible in healing skin and reclaimed childhoods — a testament to what’s possible, even if not yet permanent. It’s not a cure, but it’s a start.
“Everything that we have for the future is going to piggyback off of what we’re doing right now,” KaDee says. “Maybe tomorrow will be the day that something comes along and saves my kids’ lives.”