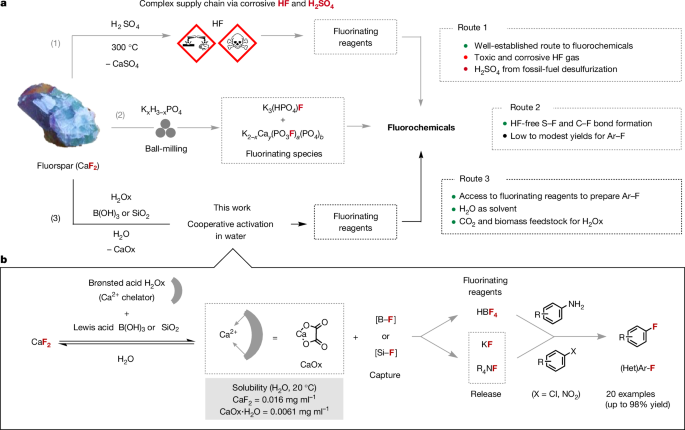
Innovations with an impact on the large-scale production of chemicals are in demand considering the pressing global challenges faced by the manufacturing industry. These include the availability and management of raw materials, transportation disruptions, complex supply chains and climate change pressure10. This urgency applies to fluorochemicals, a class of molecules that remain in increasing demand owing to their critical role as pharmaceuticals to cure diseases, agrochemicals for food security and in the production of lithium-ion batteries, which is expected to soar over the coming decade1,2,3,11. At present, the starting point of the entire fluorine industry is the treatment of fluorspar (fluorite, CaF2) with concentrated sulfuric acid (H2SO4) at high temperature (>300â°C) to produce dangerous hydrogen fluoride (HF)4,5. Subsequently, HF is used as is, or converted into various fluorinating reagents for the synthesis of fluorochemicals (Fig. 1a). For decades, other routes to HF have been studied, all featuring inorganic acids, such as hydrochloric acid (HCl), and requiring harsh reaction conditions12. We recently disclosed an activation strategy whereby fluorochemicals as diverse as sulfonyl, benzyl, allyl and alkyl fluorides can be accessed with a fluorinating reagent directly prepared from acid-grade fluorspar (AGF; >97% CaF2)13. This solid reagent obtained by ball-milling CaF2 with dipotassium phosphate (K2HPO4) consists of two crystalline components identified as K3(HPO4)F and K2âxCay(PO3F)a(PO4)b. This mechanochemical reaction stands out because it bypasses the production, storage and complex transport chain of HF. The reactivity of this fluorinating reagent for well-established transformations unavoidably requires independent investigation, and preliminary work has revealed some limitations. One of the challenges that we encountered is its broad application to the synthesis of fluoroarenes frequently used for the production of pharmaceuticals and agrochemicals. Also, mechanochemical reactions require specialized equipment that is available in only a few laboratories. This state of play encouraged the development of an alternative strategy to prepare fluorochemicals directly from fluorspar applying mild conditions for its activation. Departing from solid-state chemistry induced by mechanical energy, we surmise that activation of fluorspar in solution, and specifically in natureâs solvent water, would represent a highly attractive approach (Fig. 1b).
a, Overview of routes to fluorochemicals: (1) current industrial route, (2) preparation of fluorochemicals using phosphate-activated fluorspar, (3) synthesis of fluorinating reagents from fluorspar activated in water with oxalic acid, and B(OH)3 or SiO2. (Het)Ar, heteroaromatic. b, Cooperative activation of fluorspar using both a Brønsted acid and a fluorophilic Lewis acid (B(OH)3 or SiO2) to prepare fluorinating reagents for the synthesis of fluoroarenes (this work).
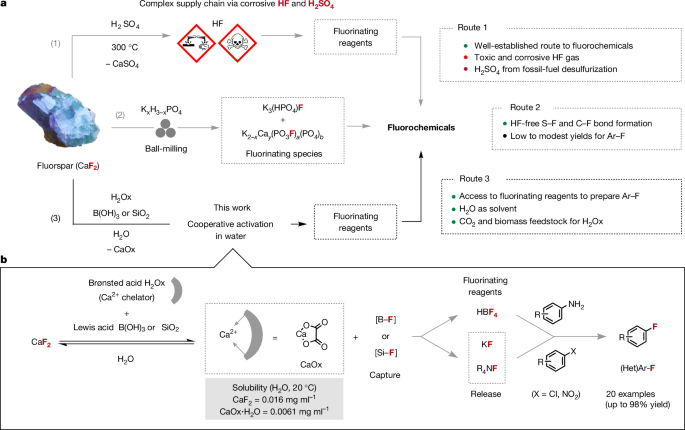
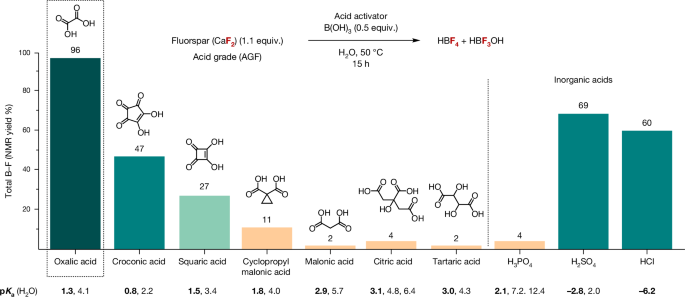
In search for a suitable manifold to activate CaF2 in water, we considered mild acidic conditions that provide direct access to frequently used fluorinating reagents other than HF. We propose that the synergistic use of a Brønsted acid and a fluorophilic Lewis acid would activate CaF2, with Ca2+ capture as an insoluble salt and simultaneous HF capture in a form suitable for subsequent fluorination processes. A report by Lennox and Lloyd-Jones provided guidance by highlighting the beneficial influence of tartaric acid on the equilibrium in solution between aryl boronic acids, potassium fluoride and potassium trifluoroborate salts through precipitation of potassium bitartrate14. This knowledge prompted us to select a suitable Brønsted acid for AGF activation that enables concurrent Ca2+ sequestration as an insoluble salt. Oxalic acid (C2H2O4, H2Ox) stood out as a suitable candidate because calcium oxalate (CaOx) is poorly soluble in water (0.0061âmgâmlâ1 (H2O, 20â°C))15,16. This choice was also driven by the appearance of economically viable and environmentally friendly methods for its synthesis, such as bio-based production in fast-growing microorganisms, or through carbon dioxide (CO2) capture6,7. The upsurge of interest in oxalic acid results from the ability of this solid organic acid to extract rare-earth elements from primary or waste ores and possibly replace existing recovery processes that depend on inorganic acids such as H2SO4 (ref. 17). Mechanistically, we surmise that the dissolution of AGF in water with oxalic acid in the presence of fluorophilic boric acid (B(OH)3) may be driven by precipitation of CaOx and immediate capture of HF as a strong BâF bond (732âkJâmolâ1) with water as a by-product15. We prioritized B(OH)3 as the Lewis acid prompted by the kinetic studies of Wamser on the reaction of hydrofluoric acid and B(OH)3 in water18, and the target BalzâSchiemann reaction for the synthesis of fluoroarenes. Fluoride capture from AGF with B(OH)3 was found to be highly effective in the presence of H2Ox when the reaction was carried out in water for 15âh at 50â°C (Fig. 2). The [BâF] products were identified by 19F and 11B nuclear magnetic resonance (NMR) spectroscopy in deuterium oxide (D2O; Fig. 3a). By 19FâNMR spectroscopy, HBF4 (quartet, chemical shift (δ) =ââ150.3âparts per million (ppm), coupling constant 1JBâFâ=â1.1âHz) and HBF3OH (quartet, δâ=ââ145.3âppm, 1JBâFâ=â10.7âHz) were identified as the major products19. In addition, a broad singlet (δâ=ââ152.3âppm) characteristic of difluoro(oxalate)borate species HOxBF2 was observed in trace amount (<1%)20. Notably, H2Ox afforded a higher yield (96%) of [BâF] products than tartaric acid (2%), H2SO4 (69%) or HCl (60%) under these reaction conditions (Fig. 2). An investigation on fluorspar activation with a range of Brønsted acids gave insight on the interplay between acidity, denticity and solubility of the Ca2+ salt by-product. For monoacids (2âequiv. versus CaF2), we noted a correlation between acidity and fluoride release in a pKa (negative logarithm of the acid dissociation constant Ka) range between 5 and â0.5 (Supplementary Table 3). Multifunctional activators (1âequiv. versus CaF2) were also investigated (Supplementary Table 4). Organic acids leading to five-membered Ca2+ chelates stood out with H2Ox (pKa1â=â1.3 and pKa2â=â4.1 (where pKa1 and pKa2 are the pKa values associated with the dissociation of the first and second proton of the diacid, respectively)) being the most effective activator followed by croconic acid (pKa1â=â0.8 and pKa2â=â2.2) and squaric acid (pKa1â=â1.5 and pKa2â=â3.4). Oxalic acid dihydrate (H2Ox·2H2O), which is more cost-effective than H2Ox, gave the [BâF] products HBF4 and HBF3OH with efficacy similar to H2Ox (total yield of 98%) upon treatment of AGF with B(OH)3 at 50â°C for 15âh (Supplementary Table 12). Subsequent studies were therefore performed with H2Ox·2H2O.
Screening of Brønsted acids for cooperative activation of AGF with B(OH)3 (2.0âmmol) at 50â°C in water. The yields of HBF4 and HBF3OH were determined by 19FâNMR spectroscopy (details in Supplementary Tables 3â6). The first pKa value (bold) refers to the dissociation of the first proton of the acid, and subsequent pKa values (non-bold) refer to the dissociation of successive protons. pKa, negative logarithm of the acid dissociation constant.
a, Preparation of HBF4 from AGF, B(OH)3 and H2Ox·2H2O with 19FâNMR (D2O) spectra of reaction mixture containing [BâF] products HBF4, HBF3OH and HOxBF2. b, Preparation of KF, NaF, CsF, Me4NF and nBu4NF from AGF, SiO2 and H2Ox·2H2O with 19FâNMR (D2O) spectra of reaction mixture containing [SiâF] products H2SiF6, H2SiF5OH and H2OxSiF4. The yields of H2SiF6, H2SiF5OH and H2OxSiF4 were determined by 19FâNMR spectroscopy (further details provided in Supplementary Fig. 18). c, Monitoring the reaction of AGF (0.5âmmol) with anhydrous H2Ox with and without B(OH)3 by 19FâNMR spectroscopy in D2O (HF, HBF4 and HBF3OH yield quantified by 19FâNMR spectroscopy; details in Supplementary Figs. 6 and 7).
It was also found that H2Ox·2H2O was a suitable activator for AGF when combined with silicon dioxide (SiO2) in water at 50â°C for 15âh, with fluoride release as [SiâF] products (SiâF bond 577âkJâmolâ1) in 97% total yield15 (Fig. 3b). The formation of H2SiF6 (broad singlet, δâ=ââ129.6âppm), which in water exists in equilibrium with H2SiF5(OH) (broad singlet, δâ=ââ128.8âppm), was evidenced by 19FâNMR spectroscopy of the reaction mixture21. Two triplets were also observed (δâ=ââ124.5âppm and â135.9âppm, 2JFâFâ=â8.9âHz) and assigned to H2OxSiF4 (supported by 29SiâNMR spectroscopy; Supplementary Figs. 14 and 15)22. Subsequent studies focused on the synthesis of frequently used fluorinating reagents from these [SiâF] products (Fig. 3b). For this purpose, AGF (1.1âequiv.) was reacted with H2Ox·2H2O (1âequiv.) and SiO2 (0.4âequiv.) in water at 50â°C for 15âh. The reaction mixture was filtered and treated with potassium hydroxide (KOH). The insoluble by-product of this filtration was unambiguously characterized as CaOx·H2O by powder X-ray diffraction (Supplementary Fig. 17). Neutralization with KOH (2âequiv.) led to the formation of K2SiF6 (Supplementary Fig. 20), whereas treatment with excess KOH (6âequiv.) afforded KFAGF (85% yield, calculated from AGF; purity analysis is included in Supplementary Information)23. A similar protocol gave NaFAGF (85% yield, 94% purity) and CsFAGF (89% yield, 96% purity) from NaOH and CsOH·H2O, respectively. Alternatively, treatment of the filtered reaction mixture with tetramethylammonium hydroxide (6âequiv.) afforded tetramethylammonium fluoride hydrate, which was converted to tetramethylammonium tert-amyl alcohol fluoride [Me4NF·(tAmOH)] AGF (88% yield), a fluorinating reagent well documented for nucleophilic aromatic fluorination (SNAr)24. This strategy also enabled the preparation of tetrabutylammonium fluoride hydrate, which was converted to the bench-stable reagent tetrabutylammonium tetra(tert-butyl alcohol) fluoride [Bu4NF·(tBuOH)4]AGF (71% yield calculated from AGF)25. Mechanistic investigations by 19FâNMR spectroscopy in D2O established whether HF is formed upon dissolution of CaF2 with H2Ox (Supplementary Fig. 6). In the absence of a fluorophilic Lewis acid, HF is indeed observed (singlet, δâ=ââ166.0âppm), and an equilibrium is established with the amount of HF plateauing after 3âh at approximately 10% (ref. 26; Fig. 3c). In the presence of either B(OH)3 or SiO2, the equilibrium is displaced via the precipitation of highly insoluble CaOx and immediate HF capture by the Lewis acid. Under these conditions, the singlet diagnostic of HF was not detected by 19FâNMR spectroscopy during the entire course of the reaction. As anticipated, the reaction of AGF with fluorophilic Lewis acid in the absence of H2Ox resulted in no fluoride release (Supplementary Information). These data highlight how Brønsted and Lewis acid cooperativity allows for fluorspar activation under mild conditions, prevents HF from building up, and enables access to HBF4 (aq.), KF, NaF, CsF, Me4NF·tAmOH and nBu4NF·(tBuOH)4, directly from fluorspar.
Further studies compared the reactivity of H2Ox·2H2O and H2SO4 with AGF. At 50â°C, the combination of AGF, concentrated H2SO4 and B(OH)3 increased the yield of [BâF] products to 97% when the reaction time was extended to 24âh (versus 69% yield after 15âh). This reaction was also effective at 25â°C, offering [BâF] products in 94% yield (average of 2 runs) after a reaction time of 48âh. For comparison, H2Ox·2H2O afforded [BâF] products in 98% yield under these conditions (25â°C, 48âh). When SiO2 served as the fluorophilic Lewis acid, activation of AGF with H2Ox·2H2O was effective at 25â°C, albeit requiring a prolonged reaction time of 72âh. After neutralization with KOH, KF was indeed isolated in 74% yield. Replacement of oxalic acid for H2SO4 did not give satisfactory results. At 50â°C for 24âh, the reaction of AGF, concentrated H2SO4 and SiO2 gave only partial fluoride release as H2SiF6 and H2SiF5OH with a total [SiâF] product yield of 48% (quantified by 19FâNMR spectroscopy). At 25â°C for 72âh, the total [SiâF] product yield was reduced to 29%. Notably, the treatment of the [SiâF] solutions with KOH afforded a solid material containing both KF and K2SO4 (46% KF (50â°C) and 36% KF (25â°C)) as evidenced by powder X-ray diffraction analysis, two salts that are challenging to separate (KF, 1,020âmgâmlâ1; K2SO4 120âmgâmlâ1 (H2O, 25â°C))15. The formation of K2SO4 is indicative of the incomplete reaction between AGF, H2SO4 and SiO2. The overall superior reactivity of H2Ox·2H2O compared with H2SO4 correlates with the solubility of the calcium by-product formed upon AGF activation (CaOx.H2O, 0.0061âmgâmlâ1 versus CaSO4.2H2O, 2.1âmgâmlâ1 (H2O, 20â°C))15.
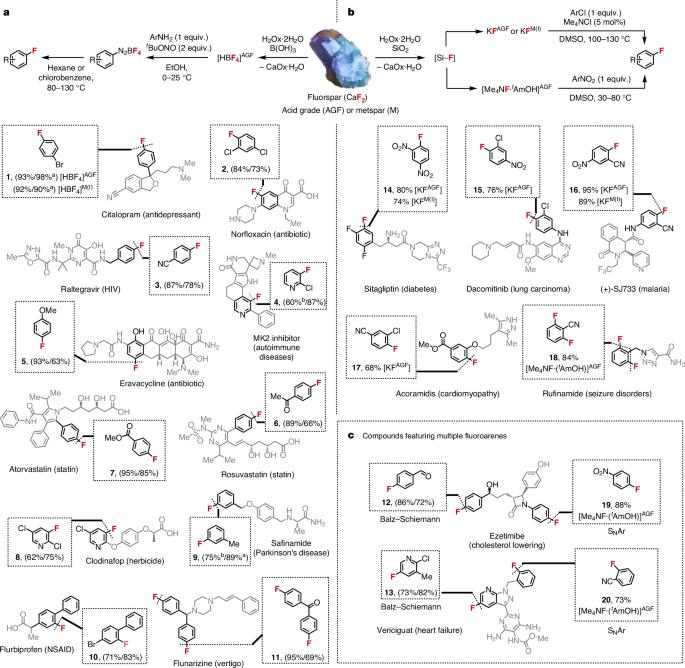
With an effective strategy to convert AGF into HBF4 (aq.), KF and Me4NF·tAmOH, we had a duty to demonstrate that these AGF-derived reagents react as expected, with the synthesis of industrially valuable fluoroarenes and a focus on those not accessible via mechanochemical activation of AGF using a phosphate salt. For BalzâSchiemann chemistry, a two-step procedure followed the preparation of HBF4 (aq.); addition of tert-butyl nitrite to a solution of aryl amine and aqueous HBF4 led to the precipitation of the corresponding aryl diazonium tetrafluoroborate salt, which was isolated and subsequently heated to liberate the desired fluoroarene27 (Fig. 4a). The protocol was validated first with 4-bromoaniline, AGF-derived HBF4 (HBF4AGF; 1.1âequiv.) and tert-butyl nitrite (2âequiv.). Heating the resulting diazonium salt at 90â°C in chlorobenzene gave 4-bromofluorobenzene in 98% yield (as measured by 19FâNMR spectroscopy), a key intermediate featured in the synthesis of the antidepressant citalopram28. This chemistry was subsequently applied to prepare multiple fluoroarenes frequently used as building blocks in the synthesis of various organo-fluorine-containing drugs in up to 87% yield (dediazoniation yield). Examples include the precursors of lipitor (cholesterol lowering), norfloxacin (antibiotic), raltegravir (HIV), eravacycline (antibiotic), rosuvastatin (cardiovascular disease), flurbiprofen (anti-inflammatory), flunarizine (vertigo) and ezetimibe (cholesterol-lowering drug)29,30,31,32,33,34,35,36. The methodology was also suitable for the preparation of fluoropyridines (4, 8 and 13) that are building blocks for drugs such as MK2 inhibitors (autoimmune diseases) and vericiguat (heart failure), and agrochemicals including the herbicide clodinafop37,38,39. For diazonium salts prone to decomposition, we developed a one-pot protocol using tert-butyl nitrite and LiBF4 enabling access to fluoroarenes (4 and 9) without the necessity to isolate the diazonium salt. For this purpose, LiBF4 was prepared from fluorspar upon treatment of HBF4AGF with Li2CO3 (Supplementary Information).
a, Fluoroarenes prepared via BalzâSchiemann reaction using AGF-derived HBF4 [HBF4]AGF or metspar-derived HBF4 [HBF4]M(I) (yield of diazotization/yield of dediazoniation). Diazotization reactions were carried out with 5.0âmmol of aryl amine, excluding compounds 3, 4, 6, 8, 9 and 11, which were carried out on 1.0âmmol of aryl amine based on differential scanning calorimetry analysis of the diazonium precursor (Supplementary Tables 29 and 30). Dediazoniation reactions were carried out on a 1.0-mmol scale (unless otherwise stated). All yields are for isolated products (unless otherwise stated). NSAID, nonsteroidal anti-inflammatory drug. b, Fluoroarenes prepared via halogen exchange (SNAr) of chloroarenes using AGF-derived KF [KF]AGF or metspar-derived KF [KF]M(I), or fluorodenitration reactions of nitroarenes using AGF-derived Me4NF·tAmOH. Reactions were carried out on a 1.0-mmol scale (unless otherwise stated). All yields are for isolated products. c, Fluoroarenes prepared using AGF-derived HBF4 for BalzâSchiemann reaction and AGF-derived Me4NF·tAmOH for SNAr reactions. M, metspar; MK, MAP-activated protein kinase. a19FâNMR yields using 4-fluoroanisole as internal standard. bDiazonium salt precursors of 4 and 9 are prepared at 10â°C (maximum process temperature <25â°C). Alternatively, 4 and 9 can be prepared via a one-pot protocol to bypass the isolation of the diazonium salt intermediate (Supplementary Information).
Next, AGF-derived KF (KFAGF) (90% purity) was found to achieve high-yielding fluorination of chloroarene substrates using Me4NCl (5âmol%) and DMSO as solvent to provide fluoroarenes 14 to 17 (Fig. 4b). We noted that the performance of KFAGF was comparable to commercial KF (99% purity; Supplementary Table 22). Aromatic fluorodenitration using AGF-derived Me4NF·tAmOH proceeded in DMSO (30â80â°C) to access 2,6-difluorobenzonitrile (18), 4-fluoronitrobenzene (19) and 2-fluorobenzonitrile (20) in high yield.
The successful cooperative activation of AGF in water and its application to the synthesis of fluoroarenes encouraged an investigation on the reactivity of lower-grade metallurgical fluorspar (metspar). These studies were performed with materials sourced from China (MetsparI CaF2 (85%), SiO2 (10%), CaCO3 (<5%), S (0.12%), P (0.1%)), and Mexico (MetsparII from CaF2 (88.98%), SiO2 (5.43%), CaCO3 (4.02%), Al2O3 (0.41%), Fe2O3 (0.24%), S (0.011%), P (0.023%), Pb (<0.001%)). Both metsparI and metsparII yielded the [BâF] products HBF4 and HBF3OH with an overall yield of 83% each, upon activation with B(OH)3 and H2Ox·2H2O at 50â°C for 15âh (Supplementary Information). The reaction of metspar with H2Ox·2H2O and SiO2 also enabled the preparation of metspar-derived KF (KFM) (53% (KFM(I)) and 63% (KFM(II)) yield, calculated from metspar I or II, respectively). Fluoroarenes 1, 14 and 16 were prepared in good yield using HBF4M(I) or KFM(I) despite the reduced purity of metspar.