Johan Paulsson’s interest in antibiotics began with body aches and nausea in August 2021. His illness, which quickly progressed to a full-body bloodstream infection, landed him in the emergency department. “It was fairly dramatic,” recalls Paulsson. He spent a week in hospital and his organs began to fail. Although his memory of the time is fuzzy, he recalls that his physicians tried three different antibiotics, one by one, to find the drug that finally made him feel better.
Even in his delirium, Paulsson — a microbial biophysicist at Harvard Medical School in Boston, Massachusetts — was alarmed that the physicians couldn’t identify the microbe behind the mystery infection. They made some guesses and tested his blood for genes of likely suspects. But there was no test that could check for any and all bacteria. And some results didn’t come back until weeks after Paulsson had got better. Even so, the tests never confirmed exactly what had made him so sick.
Paulsson thought some of the tools he’d been developing to study microbes might help. The very day he left hospital, he got in touch with colleagues to plot a solution. The result was a US$104-million project with lofty goals: to better understand how bacteria evade medicines, to develop new antibiotic candidates and to diagnose infections and antimicrobial resistance efficiently and affordably. This project, called Defeating Antibiotic Resistance through Transformative Solutions (DARTS), was launched in 2023 and is one of the first big initiatives of the US Advanced Research Projects Agency for Health (ARPA-H).
Can non-profits beat antibiotic resistance and soaring drug costs?
Penicillin was discovered nearly a century ago, and it was followed by a bevy of antibiotics derived from soil microbes, particularly Actinomyces bacteria. For a time, these drugs were helping humans to win the fight against bacterial infections.
But the well soon started to run dry, as fewer and fewer compounds were discovered. At the same time, bacteria were becoming resistant to the medications in use. Today, most new antibiotics are simply variants of a known class and can be used for just a few years before resistance emerges — not only limiting the drugs’ efficacy, but also making their development a financial loser for pharmaceutical companies. “We have to run in order to stay in place,” says Kim Lewis, a microbiologist at Northeastern University in Boston.
Hongzhe Sun, a chemical biologist at the University of Hong Kong, says that in his part of the world, “we anticipate maybe the next pandemic will be the crisis of antibiotic resistance”. Indeed, a global crisis is already happening. According to a Lancet study, about 1.27 million deaths worldwide in 2019 could be attributed to drug-resistant infections, making them a leading cause of death1. By 2050, such infections could kill as many as ten million people every year2, according to an expert panel commissioned by the UK government in 2014.
Paulsson, Lewis, Sun and others seek to give humans back the edge in the antimicrobial arms race. Some scientists aim to expedite new antibiotics or to speed up the development of accessory molecules that help antibiotics work better, using artificial intelligence (AI) and other strategies. Others hope to slow down the development and spread of resistance on the microbe side.
Researchers are optimistic that a multipronged approach can help to turn the tide (see ‘Innovation gap’). Here, Nature profiles five of the strategies that scientists are pursuing. “We may be entering an era where we can discover new antibiotics faster than resistance can evolve,” says Jonathan Stokes, a micro-biologist at McMaster University in Hamilton, Canada.
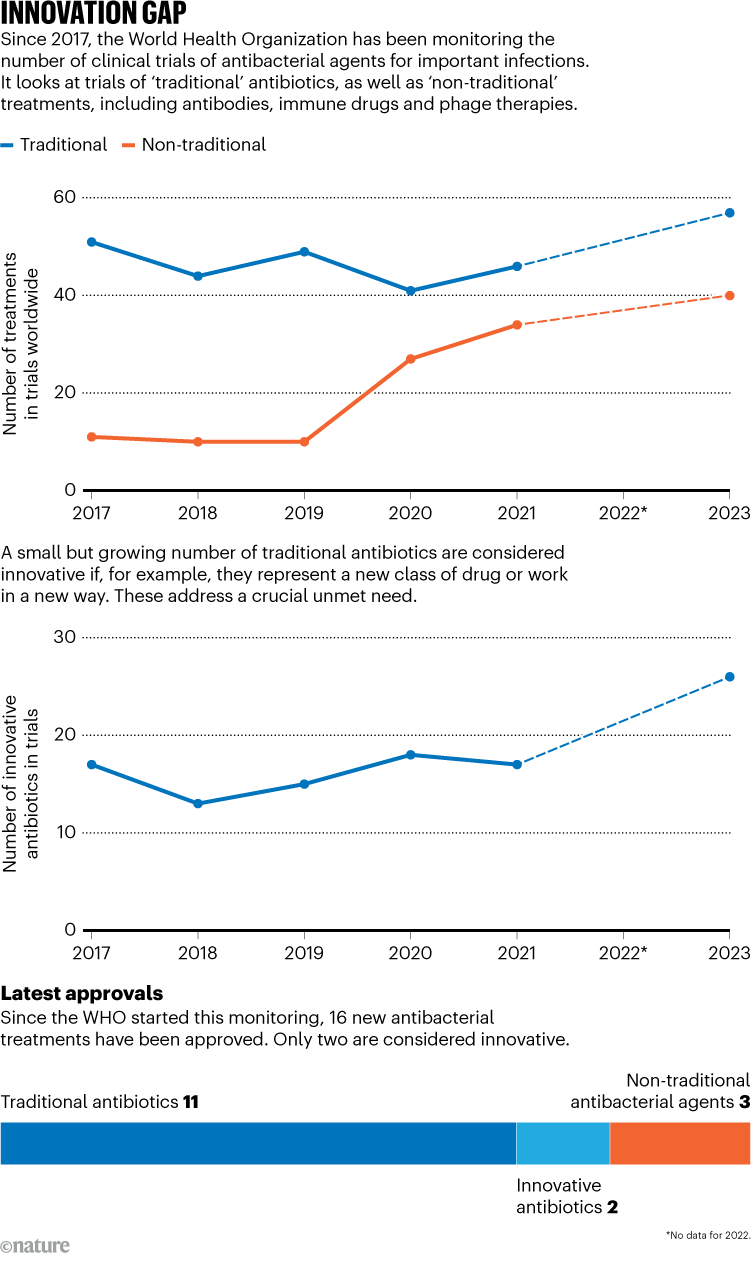
Source: WHO. 2023 Antibacterial Agents in Clinical and Preclinical Development (2024).
Natural products
Microbes still possess a lot of natural antimicrobials that scientists haven’t tapped into. For example, researchers testing Actinomyces compounds would have looked for broad-spectrum antibiotics in the past, and so might have missed molecules with a narrower target range. Lewis’s team is taking advantage of this opportunity.
Lyme disease, for one, is typically treated with broad-spectrum antibiotics that damage the healthy microbiome and promote resistance. When Lewis’s group sought Actinomyces-made compounds that would specifically kill Borreliella burgdorferi, which causes Lyme disease, the researchers rediscovered a drug called hygromycin A. First noted by researchers at the drug company Eli Lilly in 19533, it interferes with ribosomes, the protein-making machines inside cells. But the drug hadn’t been very effective, because most microbes don’t take it up. However, B. burgdorferi has a unique surface protein that grants entry to hygromycin A4. The drug is now being developed by Flightpath Biosciences in Berkeley, California, and an early-stage trial is under way, Lewis says.
Historically, microbiologists have also sought antibiotics made by the minority of bacteria that are easily grown in the laboratory. This means that a tremendous number of compounds have probably been overlooked. When Lewis and his collaborators invented a method to grow some of those hard-to-culture microbes for the first time, they found an antibiotic they called teixobactin5. The drug sticks to precursors of the bacterial cell wall and prevents them from assembling6.
Lewis co-founded Novobiotic, a company based in Cambridge, Massachusetts, to develop teixobactin and other antibiotics from species once considered impossible to culture. Teixobactin is undergoing final tests for toxicity in animals, and could go into human trials soon, Lewis says.
For his next trick, Lewis is leading the drug- discovery arm of DARTS, which is based around a microfluidics chip developed by Paulsson and other researchers. This contains millions of micro-channels that hold bacteria, all on a device about 2.5 centimetres square. By combining this with powerful automated microscopy, the researchers can watch individual pathogenic microbes as they grow and divide, and can place them alongside soil bacteria that might produce antibiotics that weaken or kill them, Lewis says.
The technology should slash the time required to identify antibiotics for further development, says Paulsson: “This method can take us there ten times faster.”
The promise of AI
Some scientists are handing antimicrobial screening over to AI. “I think AI can really help accelerate this whole thing,” says César de la Fuente, a bioengineer at the University of Pennsylvania in Philadelphia.
Many animal proteins have antimicrobial activity, and that’s what de la Fuente is hoping to harness. He’s used AI to identify short proteins, or peptides, found in modern and extinct humans, as well as other extinct animals, including woolly mammoths and giant elk7–9. It might take longer for resistance to emerge to antimicrobial peptides from extinct organisms than from modern ones, because the evolutionary pressure to resist ancient peptides would be gone by now, speculates Roby Bhattacharyya, a molecular micro-biologist at the Broad Institute in Cambridge, Massachusetts.
‘Groundbreaking’: first treatment targeting ‘super-gonorrhoea’ passes trial
But Jim Collins, a bioengineer at the Massa-chusetts Institute of Technology in Cambridge, worries that it might be difficult to convert peptides into convenient medicines because of the molecules’ large sizes. Instead, Collins and Stokes, his former postdoctoral researcher, harnessed AI to discover small molecules with antimicrobial potential. They’ve already had some successes and have co-founded a company, Phare Bio, based in Boston, to develop their ideas further.
The researchers use data from real-life experiments with antibiotics and microbes to train their algorithms to predict which molecules, out of tens of millions of known chemicals, might kill bacteria. The AI is far from perfect, says Stokes, but it’s good enough to narrow the field to just hundreds of compounds, few enough that scientists can test them in the lab.
This approach led the team first to halicin, a compound originally considered as a treatment for diabetes. Halicin interferes with the energy-producing movement of protons across microbial membranes. In the lab, it successfully treated mice infected with Acinetobacter baumannii, a pathogen that can infect lungs, wounds, blood and the urinary tract, as well as Clostridioides difficile, which causes colon infections10. The researchers also used AI to discover a compound called abaucin, which works specifically on A. baumannii11.
Now, the team has switched from using predictive AI, which considers a selection of existing molecules, to generative AI, which can devise new, potentially useful substances. The team has already begun to synthesize and test some of these12. “They are the best compounds that we’ve worked with to date,” says Akhila Kosaraju, chief executive and president of Phare Bio. With AI’s help, she predicts that the team can invent classes of antibiotics that will extend the time it takes for resistance to develop to well beyond five years.
Combination therapies
Another option is the cocktail approach, hitting microbes with many drugs at once. It’s not wholly new: the technique is already used to keep the bacterium behind tuberculosis in check, for example. But there’s still plenty of potential to find new combinations, says Nassos Typas, a systems biologist and microbiologist at the European Molecular Biology Laboratory in Heidelberg, Germany. Two drugs might work synergistically, and using a pair could even block the development of resistance to either one, he says.
A cocktail could also include molecules that aren’t microbe-killers on their own, but that help antibiotics to work better. One of the most promising ways to do this is to interfere with bacteria’s ability to communicate or group together, says Ronan McCarthy, a microbiologist at Brunel University London. Microbes join forces to secrete sticky biofilms that make them more difficult to kill, and although interfering with that process might not kill the microbes outright, it could allow antibiotics, or even immune cells, to reach the microbes and eliminate them. McCarthy and his colleagues discovered that kaempferol, a compound found in strawberries, can interfere with A. baumannii biofilms and sensitize the microbes to what would otherwise be sublethal doses of the antibiotic colistin13.
Immune assistance
New antibiotics and helper molecules can speed up the race on the medicine side, but researchers are also seeking ways to slow down the spread of resistance among microbes. One possibility is to improve clinical treatment of infection, so that fewer antibiotics are needed overall.
David Dockrell, an immunologist at the University of Edinburgh, UK, notes that the immune system handles pathogens without assistance, most of the time. Illness, Dockrell argues, results when the body’s response, such as inflammation, goes wrong.
The antibiotic paradox: why companies can’t afford to create life-saving drugs
That suggests that if physicians could ‘recalibrate’ the immune response, they could restore the body’s ability to manage the microbes. It’s the same concept as prescribing steroids, which stifle inflammation, for COVID-19.
With funding from the UK Medical Research Council, from 2016 to 2022 Dockrell led a coalition of 30 groups to research this immune-enhancing approach as a way to reduce antibiotic use. Scientists at Newcastle University, UK, for example, had previously found that when people get pneumonia after being on a ventilator, their white blood cells often have a reduced ability to ingest microbes14. The researchers are testing whether a natural immune modulator called GM-CSF might give these flagging phagocytic cells a boost. They have found that, in some individuals, it can15.
If such treatments lead to lower use of antibiotics, they’ll also reduce the pressure on microbes to evolve resistance.
Efficient diagnostics
Quick and accurate diagnosis of the cause of an infection and identification of the antibiotics that it’s sensitive to could also reduce antibiotic use and slow the evolution of resistance. “It’s actually very seldom we run into fully untreatable organisms,” says Bhattacharyya, who is also an infectious-disease physician at Massachusetts General Hospital in Boston and a DARTS collaborator. But when people are very ill and physicians can’t wait for test results, they prescribe broad-spectrum antibiotics or try multiple drugs until they succeed, as Paulsson experienced. However, trying drugs that don’t work can also speed the development of resistance.
Using Paulsson’s microfluidics-and-microscopy approach, the DARTS team is focused on how individual microbes appear — healthy, sick or dividing, for example — and how they respond to treatment, says Paul Sheehan, the project’s programme manager at ARPA-H in Washington, DC. The goal, says Sheehan, is to go from blood sample to diagnosis and antibiotic resistance profile in under an hour, which would be “a miracle”. Paulsson’s team thinks it can do this in less than ten minutes.
A similar technology, developed in Sweden, won the £8-million (US$10-million) Longitude prize on antimicrobial resistance in June for demonstrating that it can discern whether a urinary tract infection is bacterial or viral, and if bacterial, what antibiotic is most likely to work against it, all in about 45 minutes.
Diagnostics and immune modulators have the potential to protect human health as scientists learn to develop new antibiotics at speed, tilting the race back in favour of physicians and patients. And other approaches, such as vaccines and treatments based on phages — viruses that attack microbes — are also under development.
“We need more than one approach now,” says Despoina Mavridou, a microbiologist at the University of Texas at Austin. “We need more than 10 approaches, more than 100 approaches.”