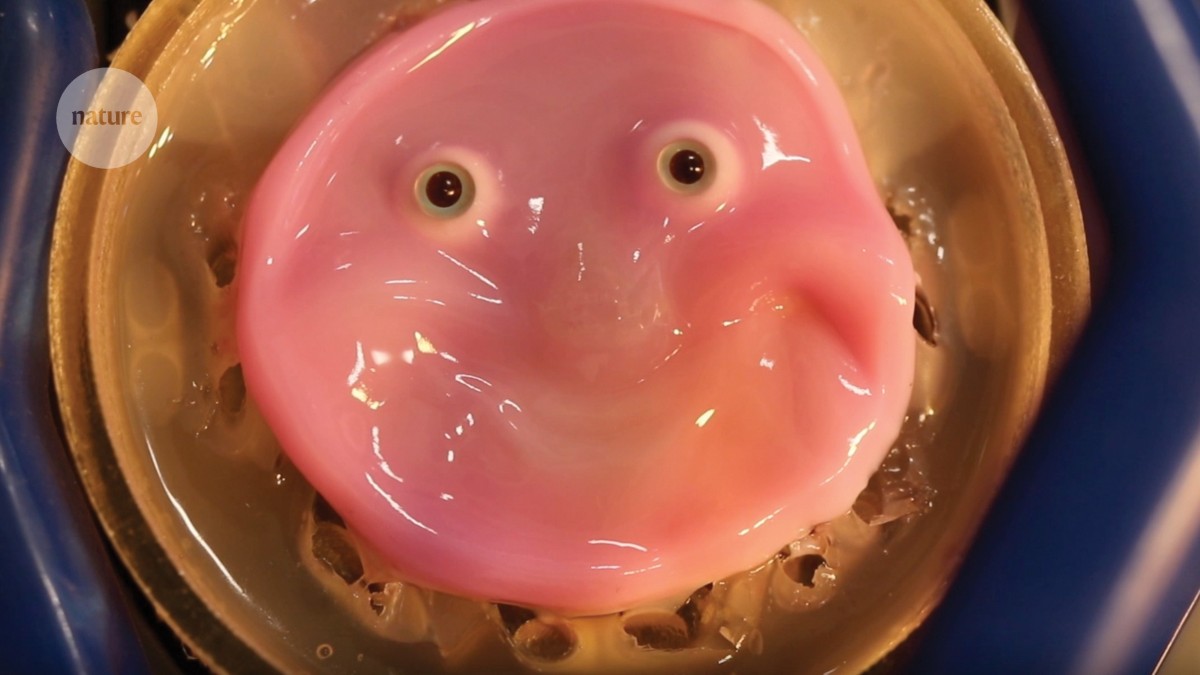
Synthetic skin, hooked to a model of a face, can form a smile.Credit: M. Kawai et al. Cell Rep. Phys. Sci. 5, 102066 (2024)
Giving robots a human face
A robot coated with living skin would look more human and, perhaps, relatable. Moreover, skin’s biological properties — especially its ability to self-repair — could make such coatings more durable than synthetic ones. One important challenge, however, is to find a way to robustly attach skin to a robot’s surface. A team at the University of Tokyo has devised a potential solution inspired by natural skin ligaments.
The group, led by engineer Michio Kawai, has previously coated robotic fingers in a simulated skin — a biomaterial that consists of an inner dermis, made of collagen and human dermal fibroblasts, plus an epidermis formed of collagen and keratinocytes. But these layers, which are applied as liquids that then solidify, were not securely attached to the robotic fingers.
Nature Outlook: Skin
To tackle this issue, Kawai’s team looked to skin ligaments — columnar extensions that the dermis sends into deeper subcutaneous tissues to attach itself to the body. To mimic these, the researchers made V-shaped tubular holes in the material to be covered. The point of each V was deep inside the material and the two tips were open to the surface. A synthetic skin — a solution of collagen and fibroblasts — was poured over and ran into the tubes. The technique created hook-like extensions on the underside of the skin that attached it to the surface.
If humanoid robots are to function well as social companions, they should be able to generate human expressions, the authors argue. To that end, Kawai and colleagues coated a model of a face with a ligament-attached dermis and grew an epidermal layer on top of this complex 3D shape. They showed that by strategically hooking their synthetic skin to a model of a mouth, the skin could remain intact and pliable, and moved with the model’s rising cheeks as the mouth formed a smile. The researchers speculate that even more lifelike expressions might be achievable by culturing muscle fibres beneath this skin.
Cell Rep. Phys. Sci. 5, 102066 (2024)
Treatment for rare fatal drug reaction
Toxic epidermal necrolysis (TEN) is a severe skin disease, usually caused by adverse drug reactions. It kills keratinocytes, causing the epidermis to detach and leading to extensive blistering. TEN arises in only about one in one million people annually — but around 15% of those affected will die from it. There is no effective treatment.
A study led by Thierry Nordmann, a skin biologist at the Max Planck Institute of Biochemistry in Martinsried, Germany, offers a potential therapy. The team uncovered a molecular pathway that drives TEN, and showed that blocking it with an existing drug might treat the disease.
The researchers took skin samples from people who experienced either TEN or milder skin reactions to drugs, and then used a technology called deep visual proteomics to catalogue thousands of proteins in keratinocytes and local immune cells. TEN-induced changes included the upregulation of proteins in an intracellular signalling network called the JAK/STAT pathway. Interferons and other inflammatory messengers that activate this pathway were also abundant. Changes occurred in both keratinocytes and immune cells, suggesting a positive feedback loop.
These findings led the authors to think that drugs that inhibit JAK enzymes might halt TEN. Tests in a mouse model proved them right — JAK inhibition drastically reduced TEN skin lesions in the animals.
Finally, the team tested their hypothesis clinically, using a drug (one already approved for a different disease) that selectively blocks the JAK1 enzyme subtype. Seven people with TEN or a slightly milder, related condition were treated; all left the hospital in good health, and none experienced side effects.
Itchy and scratchy
Scratching an itch feels good. But in people with chronic skin conditions such as eczema, the action worsens dermatitis, often setting off a frustrating cycle of scratching ever-itchier skin.
To determine what the benefits of itching might be, and to explore the mechanisms underlying these upsides, Andrew Liu, a dermatologist at the University of Pittsburgh in Pennsylvania, and his colleagues examined the interplay between immune cells and sensory neurons that links scratching and inflammation in mice. They found that scratching boosts immune responses to cutaneous bacterial infections.
Liu’s team focused on mast cells – immune cells that play a central part in allergic responses and itching. These cells, the researchers showed, interact with two classes of skin-innervating sensory neurons in a loop that links itching to scratching, then to heightened itching and greater scratching.
The study used various itch-inducing molecules to demonstrate how the activation of itch-sensitive neurons makes animals scratch affected skin. Then it showed how this scratching causes a second neuronal type (typically associated with painful sensations) to locally release a neuropeptide called substance P. This molecule interacts with the antibodies and other messengers that drive the itchy sensation, activating mast cells even further.
Finally, the researchers found that, in mice, scratching strengthens immune responses to cutaneous bacteria. It also reduced the local diversity of mice’s skin microbiome. And when the animals were given a Staphylococcus aureus infection, scratching increased the immune response. By mapping the components of the scratch response to an itch, the research suggests targets for mitigating scratching’s harmful effects.
Autonomous skin immunity
Skin is home to an abundant and diverse array of microorganisms. The immune system routinely controls this microbiota to prevent it from disrupting the skin’s function or causing systemic infections. It was long thought that this was achieved through the joint action of circulating antibody-producing B cells, T cells that recognize microorganisms directly, and antigen-presenting cells that patrol the skin. All these cells were thought to move to lymph nodes, where they interact and regulate each other’s immune functions.
But a team led by immunologist Inta Gribonika at the US National Institutes of Health in Bethesda, Maryland, found that when the skin of a mouse is colonized by bacteria that it has not previously encountered, an autonomous immune response is mounted in the skin itself. By introducing Staphylococcus epidermidis bacteria to the skin of pathogen-free mice, Gribonika and her colleagues discovered a local antibody-mediated immunity, indicating that certain B cells are the key players.
The team next showed that these B cells were activated by a population of local T cells, which transformed from an immunosuppressive phenotype into a pro-immunity one. Specialist antigen-presenting cells called Langerhans cells, which reside in the skin, were crucial to inducing this immune response. The study found that the B cells, T cells and Langerhans cells were all interacting in the skin — and not, as had been expected, in lymph nodes. ‘Tertiary’ lymphoid organs, which were previously thought to form only under inflammatory conditions, assembled around hair follicles exposed to bacteria, to coordinate local immune responses.
The researchers showed that blocking the skin’s local immune response led to unchecked growth of cutaneous populations of S. epidermidis, and that this skin-based response protects against systemic infections. Mice that exhibited this response after their skin was colonized by S. epidermidis were resistant to infections when this bacterium was later injected into the bloodstream or deeper skin layers. In showing that the skin itself mounts the first line of defence against bacteria that colonize it, the research uncovers unexpected elements of the body’s immune system.
Wearable tech monitors skin health
Unhealed skin wounds — including ones resulting from surgery or traumatic injuries, plus diabetic foot ulcers and pressure sores — are a huge medical issue. Medical professionals typically need to check such wounds regularly, to ensure that complications and infections are quickly dealt with.
Technology could enable real-time tracking of wound healing and infections outside of clinical settings. A team led by engineers Guillermo Ameer and John Rogers at Northwestern University in Evanston, Illinois, describes a small, wearable device that uses measures of water vapour, carbon dioxide and volatile organic compounds (VOCs) to monitor healing and bacterial growth.
More from Nature Outlooks
The wireless device is about the size of a Lego brick and weighs 11 grams. Pressing its concave face against the skin creates a small chamber, in which sensors track changes in the concentrations of water vapour, CO2 and VOCs as they diffuse from the surface of the body. Sensors on the device’s base measure skin temperature, thermal conductivity and electrical impedance.
To track wound healing, the device relies on the fact that lesions disrupt the skin’s barrier function, allowing more water to escape. The rate of this release wanes as healing proceeds. In tests on both healthy mice and a mouse model of type 2 diabetes, the device’s water read-outs clearly tracked wound healing, which was found to be slower in the diabetic animals.
The device could also detect infection. In mice, infected wounds produced identifiable spikes in VOCs, which are typically released by bacteria. And when human volunteers allowed their skin microbiota to accumulate, by not washing for three days, VOCs soared nearly eightfold.
The researchers report that the system can also track chemicals entering the skin, which could allow exposures to environmental toxins to be monitored. The device might be too bulky for constant monitoring of, say, foot ulcers or bedsores. But this study could mark a crucial step in the pursuit of continuous, at-home monitoring of wound healing.