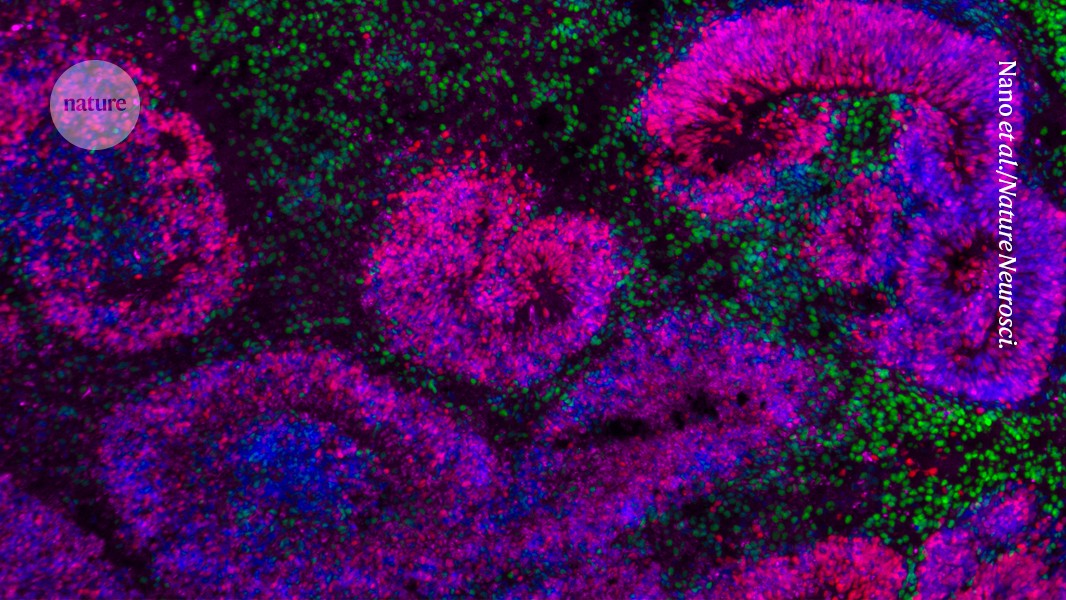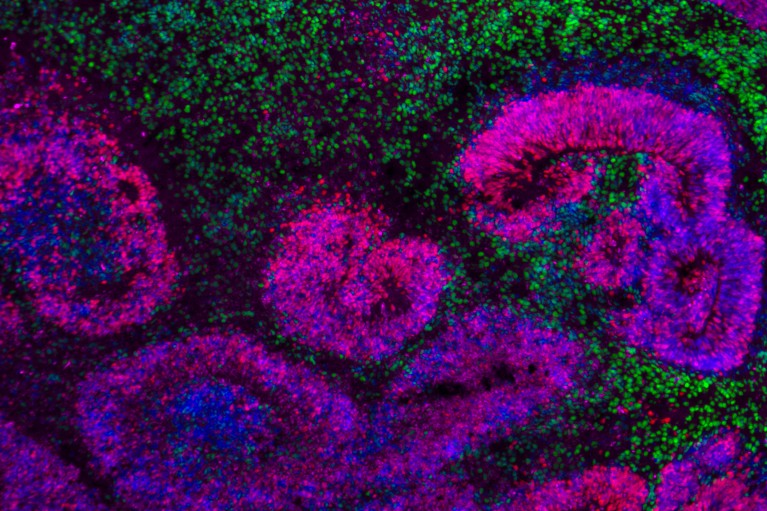
Developing human brain tissue containing rosette structures (pink and blue) surrounded by neurons (green).Credit: Nano et al./Nature Neurosci.; Jose Soto/Bhaduri Lab/UCLA
Scientists have created the most detailed maps yet of how our brains differentiate from stem cells during embryonic development and early life. In a collection of five papers published in Nature on 5 November, they tracked hundreds of thousands of early brain cells in the cortexes of humans and mice, and captured with unprecedented precision the molecular events that give rise to mixture of neurons and supporting cells.
“It’s really the initial first draft of any ‘cell atlases’ for the developing brain,” says Hongkui Zeng, executive vice-president director of the Allen Institute for Brain Science in Seattle, Washington, and co-author of two papers in the collection.
Biggest brain map ever details huge number of neurons and their activity
These atlases could offer new ways to study neurological conditions such as autism and schizophrenia, as researchers can now “mine the data, find genes that may be critical for a particular event in a particular cell type and at a particular time point”, says Zeng. “We have a very exciting time coming,” adds Zoltán Molnár, a developmental neuroscientist at the University of Oxford, UK, who was not involved with any of the studies.
The work is part of the BRAIN Initiative Cell Atlas Network (BICAN) — a project launched in 2022 by the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative at the US National Institutes of Health with US$500 million in funding to build reference maps of mammalian brains.
Patterns of development
Two of the papers map parts of the mouse’s cerebral cortex — the area of the brain involved in cognitive functions and perception.
Zeng and her colleagues focused on how the visual cortex develops from 11.5-day-old embryos to 56 days after birth. They created an atlas of 568,654 individual cells and identified 148 cell clusters and 714 subtypes1. “It’s the first complete high-resolution atlas of the cortical development, including both prenatal and postnatal” phases, says Zeng.
One surprise, she says, was how many neurons continued to take up specialised identities after birth, particularly between day 11, when newborn mice open their eyes for the first time, to day 21.
Another study focussed on brain development in human embryos. How different brain cells emerge “is a long-standing question in developmental neurobiology”, says Tomasz Nowakowski, a neuroscientist at the University of California, San Francisco. He and his colleagues created a family tree map of human cortical cells2 using brain tissue collected from 8 human fetuses at various stages of development.
Brain tissues, assemble! Inside the push to build better brain models
They identified 6,402 progenitor cells in these tissues and inserted a unique DNA ‘barcode’ into each of them. This allowed the researchers to track which stem cells generated which types of brain cells, because when the cells divide, the barcodes are copied and passed on to their daughter cells.
Nowakowski and his team found that in embryos that are less than 20 weeks old, progenitor cells were mostly developing into excitatory neurons, cells which relay electrical signals to activate other neurons. But after 20 weeks, the cells shifted to generating inhibitory neurons, ones which halt neuronal communication. “This shows how these progenitors transition from one type into the other,” says Molnár.
The team also observed that the generation of other types of brain cells — called astrocytes and oligodendrocytes — in humans “seems to be much more continuous and protracted”, compared to mice, Nowakowski adds.
“We actually don’t know what controls all these developmental programmes and switches or why they occur in development and especially at such precise moments,” says Nowakowski. Addressing these questions will be a key goal for future studies, he adds.