Human tissue acquisition
Human fetal liver tissues and cord blood samples were obtained from Tongji Hospital, Tongji University School of Medicine, Shanghai, China, with written informed consent from the parents and approval from the Medical Ethics Committee (k-w-2010-010) of Tongji Hospital. Fetal developmental age was estimated from measurements of crownârump length and compared with a standard growth chart42. Plasma from infant patients with leukaemia and benign patients was obtained from Shanghai Childrenâs Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China, with written informed consent from guardians of the patients and approval from the Medical Ethics Committee (SCMCIRB-K2024163-1) of the Shanghai Childrenâs Medical Center.
Mice
B6.129P2-Gt(ROSA)26Sortm1(DTA)Lky/J, B6.Cg-Tg(Alb-cre)21Mgn/J, B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, C57BL/6J (B6-Ly5.2) and C57BL/6JGpt-Fetua-knockout (cas9) mice were maintained and bred in a pathogen-free facility in ventilated cages, a maximum of six mice per cage, on a 12-h dayânight cycle, at 20â26â°C and 30â70% humidity, in compliance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. For embryo collection, 8â10-week-old male and female mice were mated at night and then separated the next morning; the time of separation was considered E0.5. For colony-forming cell assays, whole-genome sequencing and leukaemic models, 3-week-old mice were used. Male and female mice were used in all experiments. Mice were placed into groups depending on their gestational days and genotypes; when possible, mice were randomized and the group allocation was blinded. No sample size calculation was performed. All animal experiments were approved by the Institutional Animal Care and Use Committees of Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Isolation of human HSPCs
Liver tissues were processed immediately after isolation. The tissues were dissected into single-cell suspensions, and mononuclear cells were then separated using Ficoll density gradient centrifugation. Lineage-positive cells were depleted using the MagniSort Human Haematopoietic Lineage Depletion Kit (8804-6836-74, Thermo Fisher). Lineage-negative (Linâ) cells were incubated with a combination of biotin-labelled lineage antibodies (to CD2, CD3, CD10, CD11b, CD14, CD16, CD19, CD56, CD123 and CD235a; 8804-6836-74, Invitrogen) and FITC-labelled CD34 (581; 555821, BD). After 15âmin at 4â°C, the cells were washed with PBS, suspended in magnetic bead selection buffer (MACS; PBS, 2âmM EDTA and 0.5% BSA), and then incubated with streptavidinâphycoerythrin (12-4317-87, Thermo Fisher). After 10âmin at room temperature, the cells were washed and suspended in IMDM (12440053, Thermo Fisher) supplemented with 1% BSA. Linâ and CD34+ HSPCs were subjected to flow cytometry on an Aria III flow cytometer (BD), and the data were collected using BD FACSDiva (V8.0.3). The HSPCs were cultured in StemSpan medium (09650, Stem Cell) supplemented with 10ângâmlâ1 IL-6 (200-06, PeproTech), 10ângâmlâ1 IL-3 (200-03, PeproTech), 10ângâmlâ1 stem cell factor (SCF; 300-07, PeproTech) and 10ângâmlâ1 Flt3 (300-19, PeproTech) at a concentration of 1âÃâ105 per millilitre for further experiments.
Isolation of mouse HSPCs
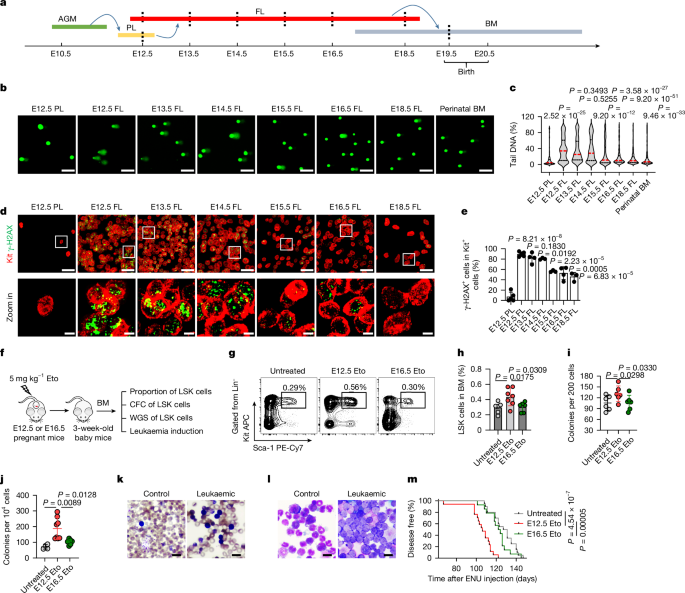
Pregnant mice were anaesthetized and euthanized by cervical dislocation. Placentas, fetal livers or bone tissues were then dissected into single-cell suspensions. Subsequently, the cells were incubated with biotin-labelled monoclonal antibodies targeting haematopoietic lineage markers (including B220, CD3, Gr-1 and Ter119; 88-7774-75, Thermo Fisher). After incubation, the cells were washed with MACS buffer and stained with streptavidin-conjugated magnetic beads (558451, BD). Following a 20-min incubation at 4â°C, the cells were washed again and resuspended in MACS buffer. Lineage-positive cells were depleted using a magnetic system. The Linâ cells were then incubated with biotin-labelled lineage markers (88-7774-75, Thermo Fisher), phycoerythrinâCy7-labelled Sca-1 (D7; 25-5981-82, Thermo Fisher), APC-labelled Kit antibodies (2B8; 17-1171-82, Thermo Fisher), phycoerythrin-labelled CD150 (mShad150; 12-1502-82, Thermo Fisher) and FITC-labelled CD48 antibodies (HM48-1; 11-0481-82, Thermo Fisher). After a 15-min incubation at 4â°C, the cells were washed with PBS and resuspended in MACS buffer. Streptavidinâphycoerythrin (12-4317-87, Thermo Fisher) or streptavidinâAPCâCy7 (405208, BioLegend) was added to the cells, which were then incubated for 10âmin at room temperature. The cells were then washed and resuspended in IMDM supplemented with 1% BSA. LSK cells, lineage-negative, Sca-l-positive, Kit-positive, CD150-positive and CD48-negative cells (LT-HSCs), lineage-negative, Sca-l-positive, Kit-positive, CD150-negative and CD48-negative cells (ST-HSCs), and lineage-negative, Sca-l-positive, Kit-positive, CD150-negative and CD48-positive cells (MPPs) were flow-sorted according the gating strategy in Supplementary Fig. 1 in the Aria III flow cytometer (BD), and the data were collected using BD FACSDiva (v8.0.3). The HSPCs were cultured in StemSpan medium (09650, Stem Cell) supplemented with 10ângâmlâ1 IL-6 (216-16, PeproTech), 10ângâmlâ1 IL-3 (213-13, PeproTech) and 10ângâmlâ1 SCF (250-03, PeproTech) at a concentration of 1âÃâ105 per millilitre for further experiments.
Isolation and culture of mouse fetal hepatocytes
To obtain Alb-Cre;ROSA26-LSL-tdTomato fetuses, we cross-mated B6.Cg-Tg(Alb-cre)21Mgn/J and B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J mice. Fetal livers were then removed at E12.5 or E16.5 and digested with 0.6âmgâmlâ1 collagenase IV (17104019, Thermo Fisher) in Hankâs balanced salt solution for 20âmin at 37â°C. The digestion reaction was halted using cold PBS, and the mixture was subsequently centrifuged at 500ârpm for 5âmin. Magnetic cell sorting (558451, BD) was used to remove blood cells expressing Ter119, B220, CD3, Gr-1 and Mac-1. Tomato-positive hepatocytes were flow-sorted using an MoFlo Astrios flow cytometer, and the data were collected using Summit (v6.3.1.16945; Beckman Coulter). The isolated hepatocytes were precultured in StemSpan medium (09650, Stem Cell) supplemented with hepatocyte growth supplement (1:100; 5201, ScienCell), 2ângâmlâ1 IL-6 and 5% FBS (F2442, Sigma; referred to as SHIF) for 4âh. After removing the medium and non-adherent cells, the adherent hepatocytes were cultured in the same medium without FBS (referred to as SHI) for 20âh. The supernatant from the cultured fetal hepatocytes (referred to as conditioned SHI or co-SHI) was collected for further experiments.
Comet assay
The HSPCs at different development stages and HSPCs cultured with or without FetuA (100âμgâmlâ1; 10318-H08H, SinoBiological) or ML216 (25âmÎ; S0469, Selleck) were treated with 20âμM etoposide (341205, Merck Millipore) for 30âmin and then collected for the comet assay. A CometAssay kit from TREVIGEN (4250-050-k, R&D) was utilized to assess DNA damage. The alkaline method was utilized using the following steps: (1) 5,000 cells were mixed with 50âµl of melted LMAgarose (4250-050-02, R&D) at 37â°C and then pipetted onto a CometSlide; (2) the cells were then gelled for 3â5âmin at 4â°C in the dark, followed by lysis with 4â°C lysis solution for 1âh; (3) the CometSlide was immersed in an alkaline unwinding solution (200âmM NaOH and 1âmM EDTA, pH > 13) for 1âh at 4â°C in the dark, and electrophoresis was conducted in the same solution at 20âV and 300âmA for 30âmin at 4â°C); (4) the slides were washed twice with ddH2O for 5âmin each, followed by washing with 70% ethanol for 5âmin, and the slides were then dried at room temperature overnight; and (5) the dried agarose was stained with SYBR Green I (A25742, Thermo Fisher) nucleic acid gel stain for 30âmin. Images were acquired with Las X (v4.7) on a Leica TCS Stellaris8 STED Microscope at Ã20 resolution and analysed using OpenComet software (v1.3.1).
Immunofluorescence staining of cells
Human HSPCs treated with 80âµM etoposide (341205, Merck Millipore) for 30âmin; mouse HSPCs treated with 20âµM etoposide for 30âmin, 500âJâmâ2 ultraviolet radiation B (UVB) irradiation; human HSPCs cultured with or without FetuA (100âμgâmlâ1; 10318-H08H, SinoBiological) for 2âh and then treated with 80âµM etoposide; mouse HSPCs cultured with co-SHI or hepatocytes for 2âh and then treated with 20âµM etoposide; and mouse HSPCs pre-treated with or without TLR4 antibody (1:50; 53-9041-80, Thermo Fisher) at 4â°C for 30âmin and then cultured with or without FetuA (100âμgâmlâ1; 50093-M08H, SinoBiological) for 2âh and thereafter treated with 20âµM etoposide were collected. These cells were then spun onto slides and fixed in 4% PFA for 10âmin. After treatment with PBS containing 2% serum, 1% BSA and 0.2% Triton X-100, the cells were directly stained with primary antibodies overnight at 4â°C. Subsequently, the sections were stained with secondary antibodies for 1âh at room temperature. Phosphor-histone H2AX (Ser13; 20E3) rabbit monoclonal antibody (1:400; 9718S) was acquired from Cell Signaling Technology (CST), and TLR4 antibody (1:200; ab13556), mouse FetuA antibody (EPR17839-163; 1:100; ab187051) and p-RPA (phospho S33; 1:200; ab211877) were obtained from Abcam. Human FetuA antibody (1F6B9; 1:100; 66094-1-Ig) was obtained from Proteintech. The MYD88 (E11; 1:400; sc-74532) antibody was acquired from Santa Cruz. Images were acquired with ZEN (v2.3) on a Zeiss 880 Microscope at Ã20 resolution or Ã63 resolution, and analysed using ImageJ (v1.52p) and HALO (v3.6.4134).
Immunofluorescence staining of whole-mount tissues
Fetal livers and placentas were fixed in 4% PFA for 30âmin, washed with PBS for 2â3âh, and stained with primary antibodies (diluted in PBS containing 1% BSA, 2% FCS and 0.5% Triton X-100) for 1â3 days. The tissues were then incubated with secondary antibodies for 2âh. Anti-mouse CD117 (Kit, ACK2; 1:50; 14-1172-85) was acquired from Thermo Fisher, and phospho-histone H2AX (Ser139; 20E3; 1:400; 9718S) rabbit monoclonal antibody was obtained from CST. Images were acquired with Las X (v4.7) on a Leica TCS Stellaris8 STED Microscope at Ã20 resolution, and analysed using Image J (v1.52p) and Imaris (v9.0.1).
Immunofluorescence staining of tissue sections
Human or mouse placenta, fetal liver and bone tissues were fixed in 4% PFA for 30â60âmin at room temperature (placenta and fetal liver tissues) or 5âh at 4â°C (bone tissues), dehydrated in 15% and 30% sucrose, and embedded in optimal cutting temperature compound at â20â°C. The tissues were then sectioned (20â25âμm) using a cryostat. The tissue sections were stained with primary antibodies for 6â12âh at 4â°C in PBS containing 1% BSA, 2% FCS and 0.5% Triton X-100. The sections were incubated with secondary antibodies for 1âh at room temperature. CD48âFITC (HM48-1; 1:100, 11-0481-82) was acquired from Thermo Fisher. APC-anti-lineage (Ter119 (1:400, 116212), Gr-1 (1:400, 108412), Mac-1 (1:400, 101212), B220 (1:400, 103212), CD3 (1:100, 100236), CD150-BV421 (SLAM; 1:100, 115925) and CD41-APC (MWReg30; 1:400, 133913)) were acquired from BioLegend. Kit goat monoclonal antibody (Gln25âThr519; Ala207Glu; 1;400, AF1356) and anti-human serum albumin antibody (MAB1455; 1:200, 188835) were acquired from R&D. E-cadherin rabbit monoclonal antibody (24E10; 1:200, 3195T) and phospho-histone H2AX (Ser139; 20E3; 1:400, 9718S) rabbit monoclonal antibodies were acquired from CST. Laminin monoclonal antibody (1:200, ab11575), mouse FetuA antibody (EPR17839-163; 1:400, ab187051) and CD34 antibody (EP373Y; 1:100, ab81289) were acquired from Abcam. CD45âFITC (104; 1:100, MCD45201) was acquired from Invitrogen. Nestin antibody (1:50, AN205-1) was acquired from Beyotime. E-cadherin antibody (DECAM-1; 1:200, sc-59778) was acquired from Santa Cruz. CD144 antibody (1:200, 550548) and Sca-1 antibody (D7; 1:200, 557403) were acquired from BD. Human FetuA antibody (1F6B9; 1:200, 66094-1-Ig) was acquired from Proteintech. Images were acquired with ZEN (v2.3) on a Zeiss 880 Microscope at Ã20 resolution or Ã63 resolution, and analysed using ImageJ (v1.52p), Imaris (v9.0.1) and HALO (v3.6.4134).
Colony-forming cell assay
The assay was performed in a semi-solid methycellulose medium (03434, Stem Cell Technologies) following the technical manual. In brief, the sorted HSPCs were plated in methycellulose in a 35-mm dish (200 cells per dish). Cultures were incubated at 37â°C in a humidified incubator (more than 95%) with 5% CO2 in the air. The colonies were scored under a microscope 10â12 days post-plating. Replating was performed by pooling total cells from primary cultures and inoculating 104 cells into fresh methycellulose medium.
Metaphase chromosome preparation and FISH
Bone marrow Linâ cells were incubated with 0.05âμgâmlâ1 colcemid for 1âh at 37â°C and then centrifuged at 400g for 10âmin. The cells were suspended in 1âml of hypotonic solution (0.075âM KCl) for 30âmin at 37â°C, and the reaction was stopped by the addition of freshly prepared fixative solution (3:1 methanol:glacial acetic acid). The cells were then subjected to three rounds of fixative changes. After that, the cells were dropped onto slides and allowed to dry at room temperature. Two-colour FISH was performed using whole-chromosome probes for mouse chromosome 4 (FITC; D-1404-050-FI, MetaSysterms) and chromosome 6 (Texas red; D-1406-050-OR, MetaSysterms), and counterstaining was performed with DAPI-Antifade solution. Images were acquired with Las X (v4.7) on a Leica TCS Stellaris8 STED Microscope at Ã20 resolution and analysed using Image J (v1.52p).
Mouse models of leukaemia
Three-week-old mice were intraperitoneally injected with 80âmgâkgâ1 N-ethyl-N-nitrosourea (ENU; N3385, Sigma) four times, which was administered twice a week, and the mice were monitored by daily observation of leukaemic symptoms and signs including fired hair, white toes, swollen lymph nodes and loss of body weight, and weekly measurement of leukaemia-like cells by Gimsa staining of peripheral blood smears. Once these appeared, the animals were killed, and the disease of leukaemia was determined by exhibition of enlarged spleens and lymph nodes, increased proportions of immature Linâ cells in the bone marrow and leukaemic cell infiltration in spleens and bone marrow shown by haematoxylin and eosin staining of the tissue sections.
Western blot
After treatment with or without FetuA (100âμgâmlâ1; 50093-M08H, SinoBiological) for 2âh, mouse HSPCs (LSK) were lysed and blotted with bZIP antibody and their phosphorylated forms. After treatment with or without FetuA (100âμgâmlâ1; 50093-M08H, SinoBiological) and the bZIP inhibitor SR11032 (2âmM; HY-15870, MedChemExpress) for 6âh, mouse Linâ haematopoietic cells were lysed and blotted with BLM antibody. The cells were lysed using SDS lysis buffer. The lysates were then separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Western blotting was carried out using the following primary antibodies: FetuA (1:2,000), JunB (1:1,000), phosphorylated JunB (p-JunB; 1:1,000), Jun (1:1,000), p-Jun (1:1,000), Fosl1 (1:10,000), p-Fosl1 (1:1,000), BLM (1:500) and laminB1 (1:2,000). The membranes were incubated overnight at 4â°C with primary antibodies. After rinsing to remove any unbound primary antibody, the membranes were exposed to a horseradish peroxidase-conjugated secondary antibody at room temperature for 1âh. The secondary antibody was detected using chemiluminescence (WBKLS0500, Merck Millipore). The following primary antibodies were used: anti-p-JunB (Thr102/Thr104; 8053S) and anti-p-Fosl1 (S265; 3880S) from CST and anti-JunB (EPR6518; ab128878), anti-Fosl1 (ab232745), anti-p-Jun (phospho S63; ab32385), anti-Jun (EP693Y; ab40766), anti-laminB1 (EPR8985; ab133741) and anti-FetuA (EPR17839-163; ab187051) from Abcam, and anti-BLM (B-4; sc-365753) from Santa Cruz. The intensity of bands was measured using ImageJ (v1.52p). For gel source data, see Supplementary Fig. 2.
Mass spectrometry analysis
SHI and co-SHI media, E12.5 and E16.5 fetal liver tissues and bone marrow plasma samples from infants were collected for data-independent acquisition tandem mass spectrometry (DIA MS/MS) analysis. The medium samples were treated with 2% SDS buffer containing 50âmM dithiothreitol for 20âmin at room temperature and then boiled at 100â°C for 5âmin. The protein samples were alkylated in the dark at room temperature for 1âh by adding 200âmM iodoacetamide. To precipitate the proteins, a 5à volume of pre-cooled acetone was used overnight at â20â°C. The tissue samples were diluted with 50âmM NH4HCO3 and centrifuged three times at 20â°C and 14,000g using YM-10 filter units. The protein lysates were reduced for 1âh at room temperature with a final concentration of 10âmM dithiothreitol and then alkylated for 1âh in the dark at room temperature with a final concentration of 55âmM iodoacetamide. The protein mixtures were exchanged with 50âmM NH4HCO3 by centrifugation at 20â°C and 14,000g three times. The protein precipitates were digested overnight at 37â°C at a protein-to-enzyme ratio of 50:1 with trypsin. Tryptic peptides were collected by centrifugation at 20â°C and 14,000g for 20âmin. The peptides were then treated with 1% trifluoroacetic acid, purified using C18 Ziptips and eluted with 0.1% trifluoroacetic acid in 50â70% acetonitrile. The eluted peptides were dried using a SpeedVac (Thermo Savant) and resuspended in 1% formic acid and 5% acetonitrile. Before analysis, indexed retention time (iRT) peptides (Biognosys) were spiked into the samples following the manufacturerâs instructions. The pooled digestates were dried using a SpeedVac (Thermo Savant) and resuspended in 5% ACN in 0.05âM ammonium formate. The digested peptides were fractionated using high-pH reversed-phase separation on a Dionex ultra-high-performance liquid chromatography (Thermo Scientific) with a 2.1âÃâ150âmm ethylene-bridged hybrid (BEH) C18 3-μm column at 40â°C, with a flow rate of 0.2âmlâminâ1 and a 60-min ACN gradient (5â30%) in 5âmM ammonium formate (pH 10). Fractions were collected at 1-min intervals and pooled at various intervals, resulting in up to 12 fractions. The samples were dried and resuspended in 1% formic acid and 5% acetonitrile. Data-dependent acquisition (DDA) analysis was conducted on an Orbitrap Fusion LUMOS mass spectrometer (Thermo Fisher Scientific) connected to an Easy-nLC 1200 via an Easy Spray (Thermo Fisher Scientific). The peptide mixtures were loaded onto a self-packed analytical PicoFrit column (75âμmâÃâ40âcm) with an integrated spray tip (New Objective) packed with ReproSil-Pur 120A C18-AQ 1.9âμm (Dr. Maisch GmbH). The peptides were separated using a 120-min linear gradient from 95% solvent A (0.1% formic acid, 2% acetonitrile and 98% water) to 28% solvent B (0.1% formic acid and 80% acetonitrile) at a flow rate of 250ânlâminâ1 at 50â°C. The mass spectrometer was operated in positive-ion mode and used the data-dependent mode with a specialized cycle time (3S) to automatically switch between MS and MS/MS scans. A full MS scan from 350 to 1,500âm/z was acquired at a resolution of Râ=â120,000 (defined at m/zâ=â400). MS/MS scans were performed at a resolution of 30,000, with an isolation window of 4âDa and higher-energy collisional dissociation fragmentation with a collision energy of 30â±â5%. Dynamic exclusion was set to 30âs. Sequences were identified using the mouse UniProt fasta database (53,099 entries, downloaded on 4 November 2018) with default parameters. The digestion enzyme used was a specific trypsin enzyme with two missed specialized cleavages. Carbamidomethyl of cysteine was set as a fixed modification, and oxidation of methionine was set as a variable modification. The iRTs derived from median iRTs across all DDA runs were calculated. Fragment ions for the targeted data analysis were selected from 300 to 1,800âm/z, with a minimal relative intensity set to more than 5% and a fragment ion number greater than 3. The false discovery rate (FDR) was set to 1% for protein and peptide spectrum matches. Protein inference was performed using the ID Picker algorithm integrated within the Spectronaut software. DIA MS/MS acquisition was performed using the same liquid chromatography-MS systems and liquid chromatography linear gradient method as DDA. For MS/MS acquisition, the DIA method was set with 50 variable isolation windows based on the full-width at half-maximum and constructed using the respective DDA data. The full scan was set at a resolution of 1,200,000 over a m/z range of 350â1,500, followed by DIA scans at a resolution of 30,000. The collision energy (CE), auto gate control (AGC) and maximal injection time were set to 30â±â5%, 1âÃâ106 and 54âms, respectively. The DIA raw files were analysed using Spectronaut X (Biognosys). The retention time prediction type was set to dynamic iRT, and a correction factor was applied for window 1. Interference correction at the MS2 level was enabled. Systematic variance was normalized using a local normalization strategy. The FDR was estimated using the mProphet approach and set to 1% at the peptide precursor and protein levels. Protein intensity was calculated by summing the intensities of their respective peptides, which were measured using the peak areas of their fragment ions in MS2 and multiplied by a factor based on the total sample volume of each sample. All the results were filtered with a Q value cut-off of 0.01 (corresponding to a 1% FDR).
Intraplacental injection of FetuA
The pregnant mice were anaesthetized using 3.5% chloral hydrate and then secured onto a heating pad with all four legs immobilized. The abdominal surface was shaved and disinfected with 75% alcohol. A longitudinal incision measuring 1â1.5âcm in length was made on the abdominal skin, and the peritoneum was cut. Cotton gauze was placed around the incision. One uterine horn was carefully exposed and pulled out using blunt forceps onto gauze soaked in PBS. The uterus was held in place with blunt forceps, and recombinant FetuA (10âμg per 15âμl each) was injected into the placenta. After the injection, the uterus was carefully returned to the abdomen, ensuring that it was positioned exactly as before. The peritoneum was closed with a haemostat. Two hours later, 5âmgâkgâ1 etoposide was administered through intraperitoneal injection. The fetuses were harvested after 1âh and fixed for immunofluorescence assays.
ATAC-seq library preparation and sequencing
ATAC-seq libraries were prepared as previously described43. In brief, HSPCs were lysed using lysis buffer containing 10âmM Tris-HCl (pH 7.4), 10âmM NaCl, 3âmM MgCl2 and 0.1% IGEPAL CA-630 for 10âmin before being spun at 4â°C to obtain nuclear preparations. The supernatant was discarded, and the nuclei were then incubated with Tn5 transposome and tagmentation buffer at 37â°C for 30âmin (Vazyme). The resulting tagmentation products were purified and amplified using PCR. PCR amplification involved ten cycles under the following conditions: 72â°C for 5âmin; 98â°C for 30âs; thermocycling at 98â°C for 10âs, 63â°C for 30âs and 72â°C for 1âmin; and 72â°C for 5âmin. The libraries were purified using a PCR purification kit (28004, Qiagen), and the fragments were enriched using 0.5à and 1.0à VAHTS DNA Clean Beads (N412-01, Vazyme) after amplification.
ATAC-seq data processing
All reads were aligned to the mm10 genome using the Burrows-Wheeler Aligner (BWA-MEM) after trimming the adapter sequences with Trim_Galore (v0.6.7). Low-quality reads were filtered out, whereas PCR duplicates and reads mapped to the mitochondria or the Y chromosome were discarded. The remaining reads on the left were shifted (+4/â5) to correct Tn5 enzyme insertions based on the read strands. Peak calling was performed using MACS2 (v2.2.6) with the following options: -f BAM, -g mm, –nomodel, –shift -100 and –extsize 200. The samples were normalized using the bamCoverage function from deepTools (v3.5.1) to visualize the signal in IGV (v2.7.0).
Quality control of ATAC-seq data was conducted, and correlation analysis was performed using deepTools (v3.5.1). The fragment distribution was generated using ATAC-seq QC (v1.14.4). The peak atlas was obtained by expanding the peak summit by ±500âbp, and differential peaks were identified using DESeq2 (v1.26.0). DNA-binding factor motifs were analysed by determining the motifs in the differential peaks using HOMER (v4.11). ATAC signals were visualized as a heatmap using the complexHeatmap package (v2.2.0).
RNA-seq and analysis
Total RNA was extracted from cultured or non-cultured HSPCs using Tri Pure Isolation Reagent (11667157001) from Roche following the manufacturerâs instructions. The quality of the RNA was assessed using the Fragment Analyser platform. High-quality samples were chosen for library construction using the Illumina TruSeq RNA Prep Kit (20015949). The libraries were subsequently sequenced on the Illumina HiSeq4000 platform, generating 2âÃâ150âbp paired-end reads. To process the raw data, Trim Galore (v0.6.7) was used with the following parameters: â–quality 20 –fastqc –length 20 –stringency 1â. The resulting clean reads were then aligned to the mouse reference genome (mm10) using hisat2 (v2.2.1). GENCODE annotations (gencode.vM25.annotation.gtf; downloaded in April 2021) and HTSeq-count (v0.13.5) were used to assign the aligned reads to genes. Subsequently, the counts were normalized to fragments per kilobase of transcript per million mapped reads (FPKM), and log(FPKMâ+â1) was utilized to analyse the overall similarity or dissimilarity between the samples.
Cut&Tag library preparation and sequencing
The Hyperactive Universal Cut&Tag Assay Kit for Illumina Pro (TD904, Vazyme) was used in this study. In summary, 1âÃâ105 LSK cells treated with or without FetuA were collected and washed in 500âµl of wash buffer. The cells were then resuspended in 100âµl of wash buffer. Subsequently, 10âµl of concanavalin A-coated magnetic beads were activated and added to 1âÃâ105 cells. The cells were incubated at room temperature for 10âmin, after which the supernatant was removed. The resulting bead-bound cells were resuspended in 50âµl of antibody buffer. Next, 1âµl of Jun rabbit monoclonal antibody (60A8; 9165T, CST), JunB rabbit monoclonal antibody (C37F9; 3753S, CST) or Fosl1 mouse monoclonal antibody (C-12; sc-28310, Santa Cruz) was added and incubated with the bead-bound cells overnight at 4â°C with rotation. The supernatant was then removed, and the bead-bound cells were resuspended in 50âµl of dig-wash buffer containing goat anti-rabbit IgG antibody (Ab207-01-AA, Vazyme) or goat anti-mouse IgG antibody (Ab208-01-AA, Vazyme; diluted 1:100). This mixture was incubated at room temperature for 1âh. The bead-bound cells were washed three times with 200âµl of dig-wash buffer to remove any unbound antibodies. Next, 2âµl of the pA-Tn5 adapter complex was diluted in 98âµl of dig-300 buffer and mixed with the bead-bound cells. The mixture was subjected to rotation at room temperature for 1âh. The bead-bound cells were washed three times with 200âµl of dig-300 buffer to eliminate any unbound pA-Tn5 protein. The cells were then resuspended in 50âµl of tagmentation buffer and incubated at 37â°C for 1âh. To terminate the tagmentation reaction, 2âµl of SDS was added to the cells and incubated for an additional 10âmin at 55â°C. The tagmentation products were purified using DNA Extract Beads Pro and eluted in 15âµl of nuclease-free water. For generation of the sequencing libraries, the DNA tagments were mixed with a universal i5 primer and a unique i7 primer and amplified using 2àCut &Tag amplification mix. The resulting PCR products were purified using VAHTS DNA lean beads (N411, Vazym), and subsequently analysed using an Agilent 2100 Bioanalyzer and Illumina Novaseq 6000.
Cut&Tag data processing
Cut&Tag reads were aligned to the mm10 genome with Bowtie2 (v2.3.5.1) using the following parameters: –end-to-end –very-sensitive –no-mixed –no-discordant –phred33 -I 10 -X 700. Duplicate reads were removed with Picard (v2.25.5). The track files were made with the bamCoverage command from deepTools (v3.5.1). Cut&Tag peaks were called using MACS2 (v2.2.6). The distribution of Cut&Tag peaks was annotated with the R package ChIPseeker (v1.22.1).
R-loop staining
HSPCs from E12.5 placenta, E12.5 fetal liver, E16.5 fetal liver or E12.5 FL-HSPCs with or without FetuA (100âμgâmlâ1; 50093-M08H, SinoBiological) or ML216 (25âmÎ; S0469, Selleck) treatment for 2âh were collected. The cells were spun onto slides, fixed in 4% PFA for 10âmin and washed three times with PBS. After permeabilization with PBS containing 0.3% Triton X-100 for 10âmin at room temperature, the cells were washed three times with PBS and then blocked with PBS containing 2% serum, 1% BSA and 0.2% Triton X-100 for 1âh at 37â°C. Immunofluorescence experiments with the dRNH1 protein were performed as previously described33. In brief, the cells were incubated with 30âμl dRNH1 (0.24âmgâmlâ1) for 1âh at 37â°C, followed by three washes with PBS. The cells were then incubated with 1âμgâmlâ1 DAPI for 10âmin. For immunofluorescence experiments with the GST-His6-2ÃHBD protein32, the cells were incubated with 30âμl GST-His6-2ÃHBD (2âμgâmlâ1) overnight at 4â°C. After three washes with PBS, the cells were stained with an anti-HisTag monoclonal antibody (AMC0149; 1:400; AE003, ABclonal) for 1âh at room temperature, followed by three washes with PBS. The cells were then stained with a rabbit anti-mouse IgG antibody (1:400; SPA231, Solarbio) for 1âh at room temperature. After three washes with PBS, the cells were incubated with 1âμgâmlâ1 DAPI for 10âmin. Images were acquired using a Zeiss 880 microscope, and the signal intensity was measured using HALO (v3.6.4134).
R-loop Cut&Tag library preparation and sequencing
The R-loop Cut&Tag library was prepared following protocols previously described32 with minor modifications. For this experiment, the Hyperactive Universal Cut&Tag Assay Kit for Illumina (TD903, Vazyme) was utilized. In brief, 1âÃâ105 cells were gently pipetted and washed twice in 500âµl of wash buffer. Then, 10âµl of concanavalin A-coated magnetic beads was activated and added to the 1âÃâ105 cells, followed by incubation at room temperature for 10âmin. The supernatant was then removed, and the bead-bound cells were resuspended in 90âµl of antibody buffer. Subsequently, 10âµl of recombinant GST-His6-2ÃHBD (0.2âmgâmlâ1) protein was added and the mixture was incubated with the bead-bound cells overnight at 4â°C with rotation. After two washes with dig-wash buffer, the samples were incubated with an anti-HisTag monoclonal antibody (AMC0149; 1:400; AE003, ABclonal) for 1âh at room temperature, followed by incubation with a rabbit anti-mouse IgG antibody (final concentration, 10âµgâmlâ1; SPA231, Solarbio) for 1âh at room temperature. Unbound antibodies were removed by three brief washes with 200âµl of dig-wash buffer. To facilitate tagmentation, 2âµl of a pA-Tn5 adapter complex was diluted in 98âµl of dig-300 buffer and mixed with bead-bound cells, which were then rotated at room temperature for 1âh. After three washes in 200âµl of dig-300 buffer to remove unbound pA-Tn5 protein, the cells were resuspended in 50âµl of tagmentation buffer and incubated at 37â°C for 1âh. The tagmentation reaction was stopped by adding 1.8âµl of 0.5âM ethylenediaminetetraacetic acid, 0.6âµl of 10% SDS, 5âµl of nuclease-free water and 1âµl of proteinase K (20âmgâmlâ1), and further incubated at 55â°C for 60âmin. Following purification with 1à DNA clean beads (Vazyme Biotech), the resulting tagmentation products were eluted in 10âµl of nuclease-free water. For the strand displacement reaction, the eluent was mixed with 10âU of Bst 2.0 WarmStart DNA polymerase (M0538, NEB) in 1à Q5 polymerase reaction buffer and incubated at 65â°C for 30âmin. The reaction was then halted by incubation at 80â°C for 20âmin. To generate the sequencing libraries, the mixture was combined with a universal i5 primer and a uniquely barcoded i7 primer and subsequently amplified using Q5 high-fidelity master mix (M0491, NEB). The libraries were size selected with 0.56â0.85à DNA clean beads and subjected to analysis using an Agilent 2100 Bioanalyzer and Illumina PE150 sequencing.
R-loop Cut&Tag data processing
The Cut&Tag data were processed as previously described32. In summary, Cut&Tag reads were aligned to the mm10 genome using Bowtie2 (v2.3.5.1), allowing for uniquely mapped reads with up to two mismatches. The aligned reads were normalized to the total number of reads (reads per million). Subsequently, track files were generated using the bamCoverage command from deepTools (v3.5.1). Cut&Tag peaks were called using MACS2 (v2.2.6), and the distribution of Cut&Tag peaks was annotated using the R package ChIPseeker (v1.22.1). The average coverage was used to create metaplots within the indicated windows. Gene Ontology enrichment analysis was performed using clusterProfiler (v3.14.3), and circus-plots were generated using circlise (v0.4.8).
Whole-genome sequencing of mouse HSPCs
Genomic DNA was extracted using the QIAamp DNA Mini Kit (51304, Qiagen) following the manufacturerâs protocol. Whole-genome sequencing was conducted as previously described44. In brief, short-insert 350-bp genomic libraries were constructed following Illumina library protocols, and sequencing was performed on an Illumina NovaSeq 6000 platform using 150-base paired-end reads, achieving an average coverage of 30Ã. The sequence data were mapped to the mouse genome reference mm10 using the BWA-MEM algorithm. Unmapped reads and PCR-derived duplicates were excluded from the analysis. Insertions and deletions (indels) and structural variants were called using the Pindel and BreakDancer algorithms, respectively, as described elsewhere45,46. The group treated with saline was used as germline control.
Cell cycle analysis of mouse HSPCs by combining Hoechst and pyronin Y
The cells were harvested from E12.5 placenta, E12.5 fetal liver and E16.5 fetal liver samples, and lineage-positive cells were depleted using a magnetic system. The Linâ cells or Linâ cells cultured with or without FetuA (100âμgâmlâ1; 50093-M08H, SinoBiological) or ML216 (25âmM; S0469, Selleck) for 2âh were collected. The cells were then suspended in 1âml of StemSpan medium (09650, Stem Cell). Hochest33342 (10âµgâmlâ1; b2261-25mg, Sigma) and verapamil (50âµM; M14204, AbMole) were added to the cell suspension. The mixture was thoroughly mixed and incubated at 37â°C for 60âmin in the dark. Subsequently, 5âµl of 100âµgâmlâ1 pyronin Y (213519-1g, Sigma) was directly added to the cells, followed by continuous incubation at 37â°C for another 15âmin in the dark. After centrifugation at 300g for 5âmin at 4â°C, the cells were suspended in MACS buffer and incubated with biotin-labelled lineage markers (88-7774-75, Thermo Fisher), FITC-labelled Sca-1 (E13-161.7; 122506, BioLegend) and APC-labelled Kit antibodies (2B8; 17-1171-82, Thermo Fisher). Following a 15-min incubation at 4â°C, the cells were washed with PBS, suspended in MACS buffer and incubated with streptavidinâAPCâCy7 (405208, BioLegend). After another 15âmin at 4â°C, the cells were washed and resuspended in MACS buffer. The samples were analysed using a Beckman cytoFLEX LX, and the data were analysed using FlowJo (v10).
DNA synthesis analysis of mouse HSPCs by EdU
Pregnant mice were administered intraperitoneal injections of 100âmgâkgâ1 EdU (CX000, CellularLab) 2âh before being killed. Cells were collected from E12.5 placenta, E12.5 fetal liver and E16.5 fetal liver and subjected to lineage-positive cell depletion using a magnetic system. The cells were then incubated with biotin-labelled lineage markers (88-7774-75, Thermo Fisher), phycoerythrinâCy7-labelled Sca-1 (D7; 25-5981-82, Thermo Fisher) and APC-labelled Kit (2B8; 17-1171-82, Thermo Fisher) antibodies. After 15âmin at 4â°C, the cells were washed with PBS, suspended in MACS buffer and incubated with streptavidinâphycoerythrin. EdU was detected using the EdU Cell Proliferation Kit with Alexa Fluor 488 (CX002, CellularLab) following the manufacturerâs instructions. The samples were analysed on a Beckman cytoFLEX LX, and the data were analysed using FlowJo (v10).
Cell cycle analysis of mouse HSCs and MPPs by Ki67
Harvested cells from E12.5 placenta, E12.5 fetal liver and E16.5 fetal liver were subjected to lineage-positive cell depletion using a magnetic system. The cells were incubated with biotin-labelled lineage markers (88-7774-75, Thermo Fisher), phycoerythrinâCy7-labelled Sca-1 (D7; 25-5981-82, Thermo Fisher), APC-labelled Kit (2B8; 17-1171-82, Thermo Fisher), phycoerythrin-labelled CD150 (mShad150; 12-1502-82, Thermo Fisher) and APCâCy7-labelled CD48 (HM48-1; 103431, BioLegend) antibodies. Following a 15-min incubation at 4â°C, the cells were washed with PBS, suspended in MACS buffer and then incubated with streptavidinâPercpcy5.5. After another 15âmin at 4â°C, the cells were fixed and permeabilized with Foxp3/Transcription Factor Staining Buffer Set Kit (00-5523-00, Invitrogen) according to the manufacturerâs instructions. Subsequently, the samples were incubated with FITC-labelled Ki67 (SolA15; 11-5698-80, Thermo Fisher). After a 30-min incubation at 4â°C, the cells were washed with permeabilization buffer and analysed using a Beckman CytoFLEX LX, and the data were analysed using FlowJo (v10).
RNA synthesis analysis of mouse HSPCs by ethyluridine
Pregnant mice were injected intraperitoneally with 50âmgâkgâ1 ethyluridine (2469â25âmg, Lumiprobe) 1âh before being killed. Cells were collected from E12.5 placenta, E12.5 fetal liver and E16.5 fetal liver and subjected to lineage-positive cell depletion using a magnetic system. The cells were then incubated with biotin-labelled lineage markers (88-7774-75, Thermo Fisher), FITC-labelled Sca-1 (E13-161.7; 122506, BioLegend) and APC-labelled Kit (2B8; 17-1171-82, Thermo Fisher) antibodies. After 15âmin at 4â°C, the cells were washed with PBS, suspended in MACS buffer and incubated with streptavidinâAPCâCy7 (405208, BioLegend). The ethyluridine was stained using the Cell-Light EU Apollo567 RNA Imaging Kit (C10316-1, RIBOBIO) according to the manufacturerâs instructions. The samples were analysed on a Beckman cytoFLEX LX, and the data were analysed using FlowJo (v10).
Statistics
Statistics analyses were performed using R (v.3.6.3) and GraphPad Prism (v.9.5). n Denotes biological replicates. For violin plots in all panels, the median and quartiles are shown. For boxplots, the meanâ±âs.d. is shown. For boxplots in Fig. 5c and Extended Data Fig. 9e,i, the median and quartiles are shown. For boxplots in Fig. 5d and Extended Data Fig. 3b, the boxes delimit the minima and maxima, and the horizontal line represents the mean. For survival analysis, the long-rank test was used to compare the difference between groups. For correlation analysis, the Pearson test was used. For comparing two groups, the unpaired Studentâs t-tests and Wilcoxon tests were used. Pâ<â0.05 was considered to be significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.