Antibodies and reagents
Antibodies
Antibodies were procured from the following: H3K36me3 (Abcam, catalogue no. ab9050), γH2AX (Millipore, catalogue no. 05-636 for IF), γH2AX (Cell Signaling Technology, catalogue no. CST9718 for western blot), pRPA2S33 (Novus Biological, catalogue no. NB100-544), pCHK1S345 (Invitrogen, catalogue no. PA5-34625), 53BP1 (Novus Biological, catalogue no. NB100-304), cyclin A (BD Biosciences, catalogue no. 611268), pRNAPII S2/S4 (Abcam, catalogue no. ab252855), pCHK1-S345 (Cell Signaling Technology, catalogue no. CST2348), CHK1 (Abcam, catalogue no. ab32531), pRPA32/RPA2-Ser8 (Cell Signaling Technology, catalogue no. 54762âS), Vinculin (Cell Signaling Technology, catalogue no. CST13901), pFGFR2-Tyr653/654 (Cell Signaling Technology, catalogue no. CST3476S) and FGFR2 (Cell Signaling Technology, catalogue no. CST11835S).
Chemicals
Chemicals were procured from the following: CHIR-124 (Selleckchem, catalogue no. S2683), XL413 (Selleckchem, catalogue no. S7547) and triptolide (Millipore, catalogue no. 645900-5MG).
Cell culture
GBM39ec, GBM39HSR and HK296 were patient-derived neurosphere cell lines and were established as previously described2,7. The parental PC3 line was obtained from ATCC. PC3 DM and PC3 HSR lines were isolated by the Mischel Lab through single-cell expansions of the parental PC3 line and are available from the Mischel Lab upon request. All the other cell lines were purchased from ATCC. Human prostate cancer cell line PC3 DM, PC3 HSR; colorectal cancer cell line COLO320DM, COLO320HSR; gastric cancer cell line SNU16; lung cancer cell line PC9 and hTERT-immortalized retinal pigment epithelial cell line RPE1 were cultured in 4.5âgâlâ1 glucose-formulated Dulbeccoâs Modified Eagleâs Medium (Corning) supplemented with 10% fetal bovine serum (FBS; Gibco). For GRO-seq and ChIPâseq, COLO320DM and COLO320HSR were grown in Roswell Park Memorial Institute 1640 with GlutaMAX (Gibco) with 10% FBS. GBM39ec, GBM39HSR and HK296 cell lines were cultured in Dulbeccoâs Modified Eagleâs Medium/F12 (Gibco, catalogue no. 11320-033) supplemented with 1à B27 (Gibco, catalogue no. 17504-01), 20ângâmlâ1 epidermal growth factor (Sigma, catalogue no. E9644), 20ângâmlâ1 fibroblast growth factor (Peprotech, catalogue no. AF-100-18B), 1â5âµgâmlâ1 heparin (Sigma, catalogue no. H3149) and 1à GlutaMAX (Gibco, catalogue no. 35050-061). GBM39 cells used in sequencing assays were cultured without additional GlutaMAX. All the cells were maintained at 37â°C in a humidified incubator with 5% CO2. Cell lines routinely tested negative for mycoplasma contamination.
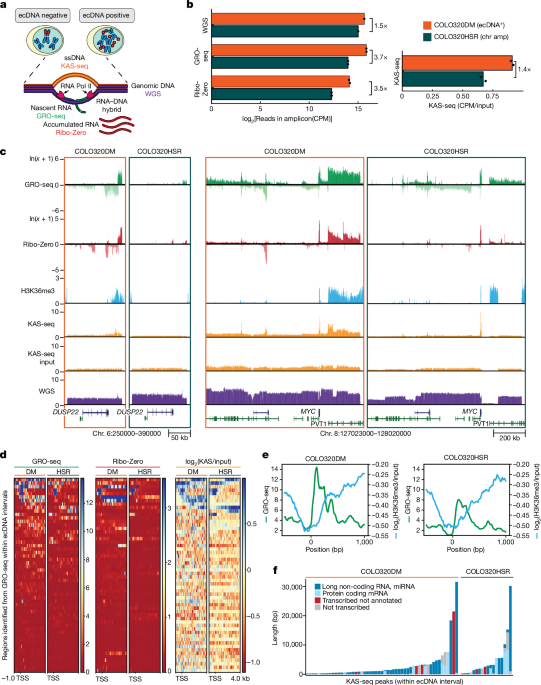
GRO-seq
COLO320DM and COLO320HSR RNA was prepared by washing cells with ice-cold phosphate-buffered saline (PBS), then adding ice-cold LB (10âmM Tris-HCl pH 7.4, 2âmM MgCl2, 3âmM CaCl2, 0.5% IGEPAL-CA630, 10% glycerol, 1âmM DTT, protease inhibitors (Roche, catalogue no. 11836170001), RNase inhibitor (Ambion, catalogue no. AM2696)) and scraping cells into a 15âml conical tube. Cells were spun at 1,000g for 10âmin at 4â°C. Supernatant was removed and pellet was thoroughly resuspended in 1âml LB using a wide bore tip. An additional 9âml LB was added and then cells were spun at 1,000g for 10âmin at 4â°C. Cells were resuspended in LB and spun down. Pellets were resuspended in ice-cold freezing buffer (50âmM Tris-HCl pH 8.3, 5âmM MgCl2, 40% glycerol, 0.1âmM EDTA, 0.2âμl RNase inhibitor per ml of freezing buffer) and spun at 2,000g for 2âmin at 4â°C. Nuclei were resuspended in 100âμl freezing buffer per 5 million cells. A nuclear run-on master mixed was prepared (10âmM Tris-HCl pH 8.0, 5âmM MgCl2, 1âmM DTT, 300âmM KCl, 0.5âmM ATP, 0.5âmM GTP, 0.003âmM CTP (unlabelled ribonucleotide triphosphates from Roche, catalogue no. 11277057001), 0.5âmM Bromo-UTP (Sigma, catalogue no. B7166), 1% Na-laurylsarcosine, 1âμl RNase inhibitor per 100âμl) and preheated to 30â°C. An equal volume of master mix was added to aliquoted nuclei (5 million nuclei per replicate) and incubated at 30â°C for 5âmin with gentle shaking. DNase digestion was performed using RQ1 DNase I and RQ1 buffer (Promega, catalogue no. M610A) for 30âmin at 37â°C; the reaction was stopped with the addition of stop buffer to a final concentration of 10âmM Tris-HCl pH 7.4, 1% sodium dodecyl sulfate (SDS), 5âmM EDTA, 1âmg mlâ1 proteinase K. Samples were incubated for 1âh at 55â°C. NaCl was added to final concentration of 225âmM. Two phenolchloroform extractions were done, followed by one extraction with chloroform. RNA was precipitated in 75% EtOH with 1âμl glycoblue (Ambion, catalogue no. 9516) overnight at â20â°C.
For GBM39ec and GBMHSR, cells were washed with ice-cold PBS and then spun for 5âmin at 500g at 4â°C. Cells were then resuspended in ice-cold 10âml swelling buffer (10âmM Tris-HCl pH 7.5, 2âmM MgCl2, 3âmM CaCl2, protease inhibitor, RNase inhibitor) and incubated on ice for 5âmin. Cell were spun at 400g for 10âmin at 4â°C and resuspended in 10âml ice-cold glycerol swelling buffer (0.9à swelling buffer, 10% glycerol). While agitating the tube, 10âml ice-cold lysis buffer (glycerol swelling buffer, 1% IGEPAL-CA630) was slowly added. Samples were incubated on ice for 5âmin, then another 25âml lysis buffer was added and samples were spun for 5âmin at 600g at 4â°C. Samples were resuspended in ice-cold freezing buffer (50âmM Tris-HCl pH 8.0, 5âmM MgCl2, 40% glycerol, 0.1âmM EDTA, RNase inhibitor) and spun at 900g for 6âmin at 4â°C. An equal volume of pre-warmed nuclear run-on master mix was added to aliquoted nuclei (10 million nuclei per replicate) and incubated at 30â°C for 7âmin with gentle shaking. Samples were then mixed thoroughly with 600âμl Trizol LS and incubated at room temperature for 5âmin. Next, 160âμl chloroform was added to each sample, shaken vigorously, then incubated at room temperature for 3âmin and centrifuged at 12,000g at 4â°C for 30âmin. NaCl was added to the aqueous phase to a final concentration of 300âmM and RNA was precipitated in 75% EtOH with 1âμl glycoblue overnight at â20â°C.
For all cell types, after overnight RNA precipitation, RNA was spun for 20âmin at 21,130g at 4â°C. RNA pellets were washed in fresh 75% EtOH, briefly air-dried and then resuspended in 20âμl water. Base hydrolysis was performed using 5âμl 1âN NaOH for 10âmin and then neutralized with 25âμl 1âM Tris-HCl pH 6.8. Buffer exchange was performed using P30 Micro columns (Bio-Rad, catalogue no. 7326250), then treated with RQ1 DNase I and RQ1 buffer and incubated at 37â°C (10âmin for COLO320 and 30âmin for GBM39). Buffer exchange was performed again. Samples were treated with 3âμl T4 polynucleotide kinase (PNK; New England Biolabs, catalogue no. M0201), 1à PNK buffer, 2âμl 10âmM ATP and 2âμl RNase inhibitor and incubated for 1âh at 37â°C. Another 2âμl PNK was added per sample and incubation was continued for 30â60âmin. RNA decapping was performed by adding ammonium chloride (final concentration 50âmM), poloaxamer 188 (final concentration 0.1%), 2âμl messenger RNA decapping enzyme (New England Biolabs, catalogue no. M0608S) and 1âμl RNase inhibitor and incubated at 37â°C for 30âmin. EDTA was then added to the final concentration of 25âmM and samples were incubated at 75â°C for 5âmin. Samples were then incubated on ice for at least 2âmin. Sample volume was then brought to 100âμl with binding buffer (0.25à SSPE, 1âmM EDTA, 0.05% Tween 20, 37.5âmM NaCl, RNase inhibitor). During T4 PNK treatment, 60âμl anti-BrdU agarose beads (Santa Cruz Biotechnology, catalogue no. sc-32323ac) per sample were equilibrated in 500âμl binding buffer by rotating for 5âmin at room temperature, spun and washed again in binding buffer. Beads were then blocked in blocking buffer (1à binding buffer, 0.1% polyvinylpyrrolidone, 1âugâmlâ1 ultrapure bovine serum albumin (BSA), RNase inhibitor) by rotating for 1âh at room temperature. Beads were then washed twice in binding buffer and resuspended in 400âμl binding buffer. Decapped RNA was then added to the blocked beads and rotated for 1âh at room temperature. Beads were then washed once in binding buffer, once in low-salt buffer (0.2à SSPE, 1âmM EDTA, 0.05% Tween 20, RNase inhibitor), once in high-salt buffer (0.2à SSPE, 1âmM EDTA, 0.05% Tween 20, 137.5âmM NaCl, RNase inhibitor) with 3âmin of rotation, and twice in Tris-EDTA-Tween20 buffer (10âmM Tris-HCl pH 8.0, 1âmM EDTA, 0.05% Tween 20, RNase inhibitor). All spins with agarose beads were performed for 2âmin at 1000g at room temperature and all washes were performed in 500âμl buffer rotating for 5âmin at room temperature unless otherwise noted. Samples were then eluted in elution buffer (50âmM Tris-HCl pH 7.5, 20âmM DTT, 1âmM EDTA, 150âmM NaCl, 0.1% SDS, RNase inhibitor) pre-warmed to 42â°C; four 10-min elutions were performed at 42â°C with periodic vortexing. The eluates for each replicate were pooled and RNA was then purified by phenolchloroform and chloroform with EtOH precipitation (COLO320) or by column purification using New England Biolabs Monarch RNA Cleanup Kit T2030 (GBM39). Sequencing libraries were prepared using the NEBNext Small RNA Library Prep Kit (New England Biolabs, catalogue no. E7330) and sequenced by Novaseq PE150. The sequence data were mapped to human reference genome (hg38) using STAR, v.2.7.10b (ref. 17). HOMER (v.4.11.1) was used for de novo transcript identification on each strand separately using the default GRO-seq setting. Reads with MAPQ values less than 10 were filtered using SAMtools (v.1.8). Duplicate reads were removed using picard-tools. GRO-seq signal was converted to the bigwig format for visualization using deepTools bamCoverage18 (v.3.3.1) with the following parameters: –binSize 10 –normalizeUsing CPM –effectiveGenomeSize 3209286105 –exactScaling.
Total RNA library preparation
Total RNA from each sample was isolated with Quick-RNA Miniprep Kit (Zymo Research, catalogue no. R1054) with input of 1â2 million cells. RNA libraries were constructed using TruSeq Stranded Total RNA Library Prep Kit with Ribo-Zero (Illumina, catalogue no. 20020596). Nextseq 550 sequencing system (Illumina) produced 20â30 million of Ã2, 75âbp paired-end reads per sample. The sequence data were mapped to human reference genome hg38 using STAR, v.2.7.10b (ref. 17), following the ENCODE RNA-seq pipeline. Reads with MAPQ values less than ten were filtered using SAMtools (v.1.8). Ribo-Zero signal was converted to the bigwig format for visualization using deepTools bamCoverage18 (v.3.3.1) with the following parameters: –binSize 10 –normalizeUsing CPM –effectiveGenomeSize 3209286105 –exactScaling.
KAS-seq library preparation
KAS-seq experiments were carried out as previously described12 with modifications13. Briefly, cell culture media was supplemented with 5âmM N3-kethoxal (final concentration), and cells were incubated for 10âmin at 37â°C in a six-well plate. Genomic DNA was then extracted using the Monarch gDNA Purification Kit (NEB T3010S) following the standard protocol but with elution using 50âµl 25âmM K3BO3 at pH 7.0. Click reaction was carried out by mixing 87.5âµl purified DNA, 2.5âµl 20âmM DBCO-PEG4-biotin (dimethylsulfoxide (DMSO) solution, Sigma, catalogue no. 760749) and 10âµl 10à PBS in a final volume of 100âµl. The reaction was then incubated at 37â°C for 90âmin. DNA was purified using AMPure XP beads by adding 50âµl beads per 100âµl reaction, washing beads on a magnetic stand twice with 80% EtOH and eluting in 130âµl 25âmM K3BO3. Purified DNA was then sheared using a Covaris E220 instrument down to around 200â400âbp size. Pulldown of biotin-labelled DNA was initiated by separating 10âµl of 10âmgâmlâ1 Dynabeads MyOne Streptavidin T1 beads (Life Technologies, catalogue no. 65602) on a magnetic stand, then washing with 180âµl of 1à Tween Washing Buffer (TWB; 5âmM Tris-HCl pH 7.5; 0.5âmM EDTA; 1âM NaCl; 0.05% Tween 20). Beads were then resuspended in 300âµl of 2à binding buffer (10âmM Tris-HCl pH 7.5, 1âmM EDTA, 2âM NaCl), sonicated DNA was added (diluted to a final volume of 300âµl if necessary) and beads were incubated for at least 15âmin at room temperature on a rotator. Beads were separated on a magnetic stand and washed with 300âµl of 1à TWB and heated at 55â°C in a Thermomixer with shaking at 1,000ârpm for 2âmin. The supernatant was removed on a magnetic stand and the TWB wash and 55â°C incubation were repeated.
Libraries were prepared on beads using the NEBNext Ultra II DNA Library Prep Kit (NEB, catalogue no. E7645). First, end repair was carried out by incubating beads for 30âmin at 20â°C in a Thermomixer with shaking at 1,000ârpm in 50âµl 1à EB buffer plus 3âµl NEB Ultra End Repair Enzyme and 7âµl NEB Ultra End Repair Enzyme. This was followed by incubation at 65â°C for 30âmin. Second, adaptors were ligated by adding 2.5âµl NEB adaptor, 1âµl ligation enhancer and 30âµl blunt ligation mix, incubating at 20â°C for 20âmin, then adding 3âµl USER enzyme and incubating at 37â°C for 15âmin (in a Thermomixer, with shaking at 1,000ârpm). Beads were separated on a magnetic stand and washed with 180âµl TWB for 2âmin at 55â°C and 1,000ârpm in a Thermomixer. After magnetic separation, beads were washed in 100âµl 0.1à TE buffer, resuspended in 15âµl 0.1à TE buffer and heated at 98â°C for 10âmin. PCR was carried out by adding 5âµl of each of the i5 and i7 NEBNext sequencing adaptors together with 25âµl 2à NEB Ultra PCR Mater Mix, with a 98â°C incubation for 30âs and 15 cycles of 98â°C for 10âs, 65â°C for 30âs and 72â°C for 1âmin, followed by incubation at 72â°C for 5âmin. Beads were separated on a magnetic stand and the supernatant was cleaned up using 1.8à AMPure XP beads.
Libraries were sequenced in a paired-end format on an Illumina NextSeq instrument using NextSeq 550 High-Output Kits (2âÃâ36 cycles). The sequence data were mapped to the hg38 assembly of the human genome using Bowtie19,20 with the following settings: -v 2-k 2-m 1–best–strata-X 1000. Duplicate reads were removed using picard-tools (v.1.99). MACS2 (ref. 21) (v.2.1.1) was used for peak-calling with the following parameters: –broad -g hs –broad-cutoff 0.01 -q 0.01. Browser tracks are generated after normalizing to input using bamCompare default setting.
ChIPâseq library preparation
Three million cells per replicate were fixed in 1% formaldehyde for 15âmin at room temperature with rotation and then quenched with 0.125âM glycine for 10âmin at room temperature with rotation. Fixed cells were pelleted at 1,300g for 5âmin at 4â°C and washed twice with cold PBS before storing at â80â°C. Membrane lysis was performed in 5âml LB1 (50âmM HEPES pH 7.5, 140âmM NaCl, 1âmM EDTA, 10% glycerol, 0.5% IPEGAL-CA630, 0.25% Triton X-100, Roche protease inhibitors 11836170001) for 10âmin at 4â°C with rotation. Nuclei were pelleted at 1,400g for 5âmin at 4â°C and lysed in 5âml LB2 (10âmM Tris-Cl pH 8.0, 200âmM NaCl, 1âmM EDTA, 0.5âmM EGTA, Roche protease inhibitors) for 10âmin at room temperature with rotation. Chromatin was pelleted at 1,400g for 5âmin at 4â°C and resuspended in 1âml of TE buffer plus 0.1% SDS before sonication on a Covaris E220 with the following settings: 140âW, 10% duly, 200 cycles per burst, 600âs per sample. Samples were clarified by spinning at 16,000g for 10âmin at 4â°C. Supernatant was transferred to a new tube and diluted with two volumes of IP dilution buffer (10âmM Tris pH 8.0, 1âmM EDTA, 200âmM NaCl, 1âmM EGTA. 0.2% Na-DOC, 1% Na-laurylsarcosine, 2% Triton X-100). Then, 50âµl of sheared chromatin was reserved as input and ChIP was performed overnight at 4â°C with rotation with 7.5âµg of H3K36me3 antibody (ab9050) (1:300 dilution). Per sample, 100âμl protein A dynabeads were washed three times with 1âml chilled block buffer (0.5% BSA in PBS) and then added to the chromatin after overnight incubation with antibody and rotated for 4âh at 4â°C. Samples were washed five times in 1âml pre-chilled wash buffer (50âmM HEPES pH 7.5, 500âmM LiCl, 1âmM EDTA, 1% IPEGAL-CA630, 0.7% Na-DOC) and then 1âml pre-chilled TEâ+â50âmM NaCl. Samples were eluted in elution buffer (50âmM Tris pH 8.0, 10âmM EDTA, 1% SDS) at 65â°C. NaCl was added to a final concentration of 455âmM. Samples were incubated with 0.2âmgâmlâ1 proteinase K for 1âh at 55â°C and then decross-linked overnight at 65â°C. Samples were treated with 0.2âmgâmlâ1 RNAase for 2âh at 37â°C and then purified with the Zymo ChIP DNA Clean & Concentrator Kit (D2505). Libraries were prepared using the NEBNext Ultra II DNA Library Prep Kit (E7645) and sequenced by NovaSeq PE150. The sequence data were trimmed by Trimmomatic22 (v.0.36) to remove adaptor and then mapped to the hg38 assembly of the human genome using Bowtie2 (refs. 19,20) with the following settings: –local –very-sensitive –phred33 -X 1000. Reads with MAPQ values less than ten were filtered using SAMtools (v.1.8). Duplicate reads were removed using picard-tools. CHIPâseq signal was converted to the bigwig format for visualization using deepTools bamCoverage18 (v.3.3.1) with the following parameters: –binSize 10 –normalizeUsing CPM –effectiveGenomeSize 3209286105 –exactScaling.
IF and DNA FISH staining
Coverslips were coated with 100âµgâmlâ1 poly-l-lysine overnight or 10âµgâmlâ1 laminin for 1âh at 37â°C before seeding cells. Asynchronized cells were seeded onto slides and subject to different treatment. Where indicated, EdU was added to each well at 10âµgâmlâ1 30âmin before collecting samples. IF and dual-IF DNA FISH staining were performed as described before. Briefly, slides were fixed with ice-cold 4% paraformaldehyde (PFA) for 15âmin, followed by permeabilization with 0.5% Triton X-100 in PBS for 15âmin at room temperature. Samples were blocked with 3% BSA in PBS for 1âh at room temperature before incubation with primary antibody diluted in blocking buffer at 4â°C overnight. Dilution ratio for first antibodies was as follows: γH2Ax, 1:500; pRPA2-S33, 1:1,000; pCHK1S345, 1:250; 53BP1, 1:500; cyclin A, 1:100; pRNAPII S2/S4, 1:1,000. After washing with PBS a total of three times for 5âmin each, slides were incubated with secondary antibody diluted in blocking buffer at room temperature for 1âh. Samples were fixed with ice-cold 4% PFA for 20âmin after washing with PBS. If combined with DNA FISH staining, fixed samples were further permeabilized with ice-cold 0.7% Triton X-100 per 0.1âM HCl (diluted in PBS) for 10âmin on ice. DNA was denatured by 1.5âM HCl for 30âmin at room temperature, followed by dehydration in ascending ethanol concentration. Diluted FISH probes (Empire Genomics) were pre-denatured at 75â°C for 3âmin and added onto air-dried slides. After incubation at 37â°C overnight, slides were washed with 2à SSC to get rid of non-specific binding, followed by DAPI staining. Where indicated, EdU staining was performed with the Click-iT Plua EdU Alexa Fluor 647 Imaging Kit (Invitrogen, catalogue no. C10640).
Validation of PC3-DM and PC3-HSR cell lines
Genomic DNA was extracted from a confluent six-well dish using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturerâs protocol. Briefly, single cells were collected and resuspended in 200âµl 1à PBS, followed by the addition of 20âµl QIAGEN proteinase K and 200âµl buffer AL. The mixture was pulse-vortexed for 15âs and incubated at 56â°C for 10âmin. A volume of 200âµl absolute ethanol was added to the sample and pulse-vortexed for 15âs. The entire mixture was pipetted into a QIAamp Mini spin column and centrifuged at 6,000g for 1âmin. Filtrate was discarded and 500âµl buffer AW1 was added to the column. After centrifugation at 6,000g for 1âmin, the column was subjected to another round of wash with 500âµl buffer AW2. The filtrate was discarded after centrifugation at full speed for 3âmin. The column was then placed in a clean 1.5âml microfuge tube and 50âµl of buffer AE was added to reconstitute genomic DNA after centrifugation at 6,000g for 1âmin.
WGS library preparation was performed with the FS DNA Library Prep Kit from NEB according to the manufacturerâs protocol, with these parameters in place: (1) 250âng gDNA was used as input; (2) fragmentation was done with an incubation time of 18âmin to yield 200â450âbp fragments; (3) the final library size distribution was between 320â470âbp (that is, first bead selection was done with a bead volume of 30âµl and second bead selection was done with a bead volume of 15âµl); (4) the final PCR amplification was performed for four cycles. PE150 sequencing was performed on NovaSeq to yield at least 10à coverage at Novogene. Adaptor sequences were removed from raw fastq files using Trim Galore at default settings, followed by alignment to the hg38 reference genome using Map with BWA-MEM to generate the BAM files. BAM files were then uploaded to the GenePattern Notebook for AmpliconArchitect analysis under default settings.
ecDNA structure analysis
We utilized the AmpliconSuite-pipeline (v.1.2.2, https://github.com/AmpliconSuite/AmpliconSuite-pipeline), which invoked CNVKit (v.0.9.9)43, AmpliconArchitect44 (AA; v.1.3.r8) and AmpliconClassifier3 (AC; v.1.1.2). In brief, the analysis pipeline first identifies seed regions of focal amplification from whole-genome copy number calls, then among the seed regions AA analyses copy number and structural variation jointly to construct a local genome graph encoding structural rearrangements and copy numbers. AA then extracts genome paths and cycles from the genome graph that explain the observed changes in copy number and structural variation. The outputs of AA are passed to AC, which applies a rule-based method to match the patterns of copy number, structural variation and structures extracted from the genome graph to known types of focal amplifications, such as ecDNA. To minimize sequencing artefacts derived from insert size distribution variance, we set the AmpliconSuite-pipeline argument –AA_insert_sdevs 9. For PC3 samples, –downsample 1 was also set to reduce additional sequencing artefacts. Default parameters were used otherwise.
For COLO320DM/HSR, we utilized the general ecDNA regions and the candidate ecDNA structure from ref. 10, after lifting over coordinates to hg38. For GBM39ec/HSR and PC3-DM/HSR, ecDNA regions were derived from AA output files. From the DM samples, regions with copy number greater than ten in the AA amplicon containing the ecDNA of interest were defined as the ecDNA regions. In GBM39ec/HSR, we also included the vIII deletion in the ecDNA region. Candidate ecDNA structures were derived from the AA cycle with highest assigned copy count containing the oncogene of interest (GBM39: EGFR and PC3: MYC). For GBM39, the ecDNA structure was consistent with a previously published reconstruction11. Circular ecDNA visualizations were generated with CycleViz (https://github.com/AmpliconSuite/CycleViz). Gene and focal amplification copy numbers were derived from the AA graph file and the AC feature basic properties file, respectively. Structural similarity scores of the focal amplifications were computed using the feature_similarity.py script in AC, which computes a similarity score based on the overlapping genomic boundaries and shared structural variants between two focal amplifications. For the PC3 samples, we utilized the related amplicon_similarity.py script to obtain similarity scores, as the exact boundaries of the ecDNA could not be easily resolved with AC.
Replication combing assay
Replication fork speed in ecDNA was evaluated using the molecular combing assay. COLO320DM and COLO320HSR cells were seeded into plates and allowed to grow into log phase, nascent DNA synthesize was pulse labelled with thymidine analogues: CldU and IdU sequentially for equal amount of time. Following pulse labelling, cells were harvested and embedded into agarose plugs using the Genomic Vision FiberPrep Kit (Genomic Vision). DNA extraction, combing and immunostaining was performed according to the EasyComb service procedures (Genomic Vision). Coverslips were scanned with a FiberVision scanner and images were analysed using FiberStudio software (Genomic Vision). Fork speed was calculated using replication signals with contiguous CldUâIdU tracks. Only intact signals, flanked by counterstaining of the DNA fibre, were selected for analysis.
Locus-replication combing assay
DNA replication activity at the MYC loci was assessed using molecular combing assay. COLO320DM and COLO320HSR cells were seeded into plates and allowed to grow into log phase, nascent DNA synthesize was pulse labelled with thymidine analogues: CldU and IdU for equal amount of time. Following pulse labelling, cells were harvested and embedded into agarose plugs using the Genomic Vision FiberPrep kit (Genomic Vision). DNA extraction and combing was performed according to the EasyComb service procedures (Genomic Vision). DNA-labelled FiberProbes (Genomic Vision) targeting MYC loci were produced and hybridized to combed DNA. Correspondence between theoretical and experimental probe coverage patterns was validated by measuring hybridized probe length in control samples. After immunostaining of replication signals and DNA probes, coverslips were scanned with a FiberVision scanner. Image analysis and measurements were performed using FiberStudio software (Genomic Vision). Fork speed was calculated using replication signals with contiguous CldUâIdU tracks.
Comet-FISH
Alkaline comet-FISH assays were performed according to the literature, with minor modifications45,46. Cells were harvested by trypsinization, washed with PBS and placed on ice. Cells were diluted in 37â°C low melting point (LMP) agarose (IBI Scientific) in PBS to a final concentration of 0.7% and spread on precoated glass slides with a coverslip. Overnight lysis was performed at 4â°C in alkaline lysis solution (2.5âM NaCl, 100âmM EDTA, 10âmM Tris pH 10, 1% Triton X-100, 10% DMSO) protected from light. The following day, slides were equilibrated for 30âmin in alkaline electrophoresis buffer (200âmM NaOH, 1âmM EDTA, pH less than 13) in a Coplin jar and subsequently electrophoresed at 25âV for 30âmin. Slides were then neutralized with Tris, dehydrated in 70% ethanol and dried at room temperature.
To detect ecDNA through FISH, Cy5-labelled probes were generated from RP11-440N18 BAC DNA sonicated to 150âbp and labelled using a DNA labelling kit (Mirus Bio). Slides were denatured with 0.5âM NaOH for 30âmin at room temperature, dehydrated in an ethanol series (70%, 85%, 95%) and allowed to dry at room temperature. The hybridization mixture containing probe DNA (200âng per slide) and Cot-1 DNA (8âμg per slide) was denatured separately at 75â°C for 10âmin and pre-annealed at 37â°C for 1âh. Probe was added to the slides and spread with a glass coverslip and incubated at 37â°C overnight in a humidified chamber. The following day, slides were washed four times in 2à SSC, 50% formamide at 42â°C and subsequently washed twice in 2à SSC at 42â°C. Slides were dipped briefly in 70% ethanol and air-dried. Slides were mounted with Everbrite (Biotium) containing SYBR Gold (Invitrogen) diluted 1:10,000 and sealed with nail polish. Images were collected on a Nikon Eclipse TE2000-E using a Ã60 oil objective.
Cell viability assay
Cell viability assay was performed using CellTiter-Glo (Promega, catalgoue no. G8461) as previously described47. Briefly, cells were seeded into a 384-well plate one day before adding inhibitors. Equal volumes of vehicles or drugs diluted at indicated concentration were added into each well the next day, and the cells were incubated for three days. On the third day, after equilibrating plate and CellTiter-Glo reagent at room temperature for 30âmin, reagent was added into each well and incubated for 15âmin at room temperature. Luminescence was measured using a Synergy 2 microplate reader. Four biological replicates were performed for each condition. Data analysis was performed with GraphPad Prism (v.9.1.0).
TUNEL
TUNEL assay (Invitrogen, catalogue no. C10617) was performed to detect DNA fragmentation during apoptosis. COLO320DM, COLO320HSR and SNU16 cells were treated with 1âµM CHIR-124 for indicated times. All cells including floating cells were collected and spun down onto slides using a cytospin (Thermo Scientific Cytospin 4 Centrifuge). Slides were fixed with 4% PFA and permeabilized with 0.25% Triton X-100, followed by labelling of free double strand end with EdUTP by reaction catalysed by TdT enzyme in a humidified chamber at 37â°C for 60âmin. Incorporated EdUTP was detected through Click-iT Plus TUNEL reaction according to the manufacturerâs manual at 37â°C for 30âmin. Slides were counterstained with DAPI and mounted with ProLong Diamond Antifade.
Annexin V staining
Cell apoptosis was detected through flow cytometry using a FITC Annexin V Apoptosis Detection Kit (BD Biosciences, catalogue no. 556547). Cells were treated with 1âµM of CHIR-124 for the indicated time, and all the cells including floating cells were collected. After washing with PBS twice and cell number counting, cells were resuspended in 1à binding buffer provided by the kit at a concentration of 1âÃâ106âcells per millilitre. One hundred microlitres of the cell suspension was transferred to a FACS tube and stained with FITC Annexin V and propidium iodide. After incubation at room temperature for 15âmin, all the samples were analysed with BD LSR II flow cytometry (BD Biosciences) within 1âh. Flow cytometry data were analysed through Beckman Coulter Kaluza software (v.2.1).
Microscope and image analysis
Images were taken by conventional fluorescence microscopy or confocal microscopy. Conventional fluorescence microscopy was performed on a Leica DMi8 widefield microscope by Las X software (v.3.8.2.27713) using a Ã63 oil objective. Confocal microscopy was performed on a ZEISS LSM 880 inverted confocal microscope using ZEN (black v.2.3) (Stanford CSIF Facility). Z-stacks were taken for each field of view and a best-in-focus stack was identified for downstream image analysis, except for Fig. 3a, where a max projection was performed by ImageJ (v.1.53t).
Image analysis and quantification were performed using the open-source software CellProfiler (v.4.2.1). For foci number analysis, DAPI staining, IF staining and DNA FISH channel were analysed through automatic thresholding and segmentation to cell nuclei, pRPA2S33/γH2AX foci and DNA FISH foci respectively. Colocalization was performed using an object-based colocalization method. For fluorescence intensity measurement, nuclei were called based on DAPI channel through automatic thresholding and segmentation; mean fluorescence intensity was retrieved by measuring mean fluorescence intensity within each nucleus.
RS score computation
RS score 1
The gene set variation analysis48 was utilized to assess the enrichment of the DNA RS response (RSR) gene set20 in TCGA samples using RNA-seq data49. The RSR gene set was curated based on genes affected by defects in the DNA RS response. RNA-seq transcripts per kilobase million values for TCGA samples were retrieved from the GDC data portal49. Gene set variation analysis generated enrichment scores for both up- and down-regulated RSR genes. The final RSR score was determined as the difference between the up and down enrichment scores.
RS score 2
The RS signature score of each sample from TCGA was retrieved from the literature from ref. 21, which was transformed linearly between zero and one by subtracting the minimum score and dividing by the maximum score. TCGA sample ecDNA status classification was performed as stated in a previous publication1.
Both methods
Briefly, 1,921 TCGA samples were grouped into five subtypes by AC (https://github.com/AmpliconSuite): ecDNA, breakageâfusionâbridge, complex non-cyclic, linear and no amplification. Samples with a breakâfusionâbridge or complex non-cyclic status were removed from the analysis due to the challenges of detecting ecDNA from short-read data. Samples with linear amplification and no amplification were classified as ecDNAâ. After removing metastasis sample and ecDNAâ samples without matching ecDNA+ samples of the same tissue origin, a total of 232 ecDNA+ and 582 ecDNAâ samples were included in the analysis.
CRISPR experiment
sgRNA template oligos targeting the gene encoding CHK1 was synthesized (Integrated DNA Technologies) and was ligated into a CRISPR expression vector with red fluorescent protein (RFP) (Cellecta-pRSG16-U6-sg-HTS6C-UbiC-TagRFP-2A-Puro). Non-targeting green fluorescent protein (GFP) (sgNT-GFP) plasmid was purchased.
ecDNA+ and ecDNAâ Hela cells were transduced with sgCHK1-RFP or sgNT-GFP virus, and puromycin (Sigma) was added at 2.5âµgâmlâ1 for selection for 48âh. After 48âh of puromycin selection (day 0), an equal number of cells expressing either sgCHK1-RFP or sgNT-GFP were mixed to obtain the RFP to GFP population ratio. In the following days, flow cytometry analysis was performed to determine the sgCHK1-RFP to sgNT-GFP ratio. The mixed cell population cultures were maintained at subconfluency. The sgRNA sequences targeting CHK1 were as follows:
No. 17: CCTGACAGCTGTCACTGGGT
No. 18: GCTGTCAGGAGTATTCTGAC
Western blotting
Samples were lysed in radioimmunoprecipitation assay buffer (Boston BioProducts, catalogue no. BP-115) supplemented with protease/phosphatase inhibitors (Fisher Scientific, catalogue no. 78444). Protein concentration was quantified with bicinchoninic acid assay (Fisher Scientific, catalogue no. 23225) and samples were prepared in 4à sample buffer (Bio-Rad, catalogue no. 1610747). Samples were loaded and run on 4â12% Bis-Tris Gradient Gel (Fisher Scientific, catalogue no. WG1403BOX) and transferred onto a nitrocellulose membrane (Bio-Rad, catalogue no. 1704271). The membrane was blocked with 5% BSA in Tris-buffered saline with Tween (Fisher Scientific, catalogue no. 28360) for an hour, and then primary antibody (1:1,000 dilution) was added and incubated overnight at 4â°C. Following primary antibody incubation, the membrane was washed with Tris-buffered saline with Tween and incubated with secondary antibody for 1âh. The membrane was then incubated with enhanced chemiluminescence reagent (Fisher Scientific, catalogue no. 32106) and image acquisition was performed on ProteinSimple FluorChemE.
Detection of phosphorylated CHK1 Ser345 using the AlphaLisa SureFire assay
Compound activity in cells was measured using an AlphaLISA SureFire Ultra p-CHK1 (Ser345) assay (Perkin Elmer, catalogue no. ALSU-PCHK1-A10K). HT29 cells were cultured in McCoy 5âA medium with 10% FBS and 1% penicillin-streptomycin and seeded to 96-well plates (Corning, catalogue no. 3599). Compounds were serially diluted in DMSO over a 10-point dose range with 3-fold dilution, and compound solution was added to each well containing cells. Plates were centrifuged at 1,000ârpm for 30âs. Plates were incubated at 37â°C for 16âh. Supernatant was removed by flicking the plate against a paper towel. Wells were washed once with PBS solution. To each well was added freshly prepared lysis buffer and plates were agitated on a plate shaker at 400ârpm for 30âmin. The 96-well cell plates were centrifuged at 1,500ârpm for 1âmin. From each well was transferred 10âµl of the lysates to a 384-well Optiplate (Perkin Elmer, catalogue no. 6007290). To each well was added Acceptor Mix (5âµl) and the plates were sealed and wrapped in foil. Plates were agitated on a plate shaker for 2âmin, then incubated at room temperature for 1âh. To each well was added Donor Mix (5âµl) and the plates were sealed and wrapped in foil. Plates were agitated on a plate shaker for 2âmin, then incubated at room temperature for 1âh. AlphaLisa signal was read on an EnVision multimode plate reader (Perkin Elmer). Data were fitted to doseâresponse curves using XLfit (IDBS) or GraphPad Prism (GraphPad software) to calculate IC50 values for each compound tested.
Kinase HTRF biochemical assay
CHK1 enzyme activity was measured using a homogeneous time resolved fluorescence (HTRF) KinEASE assay (Cisbio, catalogue no. 62ST1PEC). Full-length human CHK1 protein (GenBank accession number NP_001265.1) was obtained from Carna Biosciences, Inc. (catalogue no. 02-117). The enzyme reaction was carried out in assay buffer containing (final concentrations): CHK1 enzyme (0.012ângâµlâ1), MgCl2 (5âmM) and DTT (1âmM). To determine compound dose response, DMSO stock solutions were serially diluted in a ten-point concentration series in duplicate. Compound solution (50ânl) was added to 384-well assay plates (Greiner, catalogue no. 784075). To each well containing compound solution was added assay buffer solution (5âµl). Plates were centrifuged at 1,000ârpm for 1âmin, then incubated at room temperature for 10âmin. The reaction was started by addition of substrate buffer (5âµl per well) containing (final concentrations): STK substrate 1-biotin (120ânM) and ATP (1âmM). Assay plates were centrifuged at 1,000ârpm for 1âmin, then incubated at room temperature for 60âmin. The reaction was stopped by the addition of detection buffer (Cisbio, 10âµl) containing (final concentrations): STK antibody cryptate (0.25ânM) and streptavidin-XL665 (7.5ânM). Plates were centrifuged at 1,000ârpm for 1âmin, then incubated at 25â°C for 2âh. HTRF signal was read on an EnVision multimode plate reader (Cisbio) in HTRF mode. Data were fit to doseâresponse curves using XLfit (IDBS) or Prism (GraphPad Software) to calculate IC50 values for each compound tested.
Phospho-RPA32 S8 IF high content imaging
Optical-bottom 96-well plates (Thermo Scientific, catalogue no. 165305) were coated with 50âµl of 1:1 poly-l-lysine (R&D Systems, catalogue no. 3438-100-01) and poly-d-lysine (R&D Systems, catalogue no. 3439-100-01) for 3âh at room temperature. The wells were washed once with 100âµl of PBS (Gibco, catalogue no. 10010-023) and all liquid was removed from the wells and allowed to dry fully at room temperature. COLO320 ecDNA+ cells were seeded at 15,000 cells per well in 100âµl of Roswell Park Memorial Institute media (Thermo Fisher, catalogue no. 22400089) supplemented with 10% FBS (Omega Scientific, catalogue no. FB-01). Cells were left to attach in a 37â°C incubator with 5% CO2 overnight. The following day, cells were treated with BBI-825 for 16âh. Following treatment, all culture media was removed, and cells were fixed with 4% PFA (Boston BioProducts, catalogue no. BM-155) for 15âmin at room temperature. After fixation, the 4% PFA was removed and wells were washed twice with 100âµl of PBS. The cells were then permeabilized with 100âµl of 0.5% Triton X-100 (Sigma-Aldrich, catalogue no. T8787) in PBS for 15âmin at room temperature. After permeabilization, wells were washed twice with 100âµl of PBS and then blocked with 5% goat serum (Abcam, catalogue no. ab7481) and 1âmgâmlâ1 of BSA (GeminiBio, catalogue no. 700-100 P) for 1âh at room temperature. The primary antibody (phospho-RPA32 (S8); Cell Signaling, catalogue no. 54762) was diluted at 1:200 in blocking buffer and 50âµl was added to all wells and incubated at 4â°C overnight. Plates were then washed three times with 100âµl of PBS and then incubated with 1:1,000 dilution of secondary antibody (Goat anti-Rabbit IgG Alexa Fluor Plus 594; Thermo Fisher, catalogue no. A32740s) and 1:1,000 dilution of Hoechst 33342 (Biotium, catalogue no. 40046) in blocking buffer for 1âh at room temperature. Plates were then washed three times with 100âµl of PBS; 100âµl of PBS was left in the wells following the final wash. The plate was imaged using a CellInsight CX7 LZR Pro High Content imager (Thermo Fisher Scientific) and data analysed using the Spot Detector BioApplication module on the HCS Studio Cell Analysis software (Thermo Fisher Scientific). Puncta were detected using a pixel thresholding method within a nucleus, and cells that contained three or more puncta of phosphorylated RPA32 Ser8 staining were considered as a positive signal.
Xenograft
Animal experiments were performed in accordance with protocols approved by the Charles River Accelerator and Development Lab (CRADL) Institutional Animal Care and Use Committee (protocol no. EB17-010-066). Mice were socially housed in individually ventilated cages on a 12/12âh light/dark cycle with temperatures between 65 and 75â°F and 30â50% humidity. The SNU16 gastric cancer cell line was purchased from ATCC (catalogue no. CRL5974) and maintained in Roswell Park Memorial Institute growth medium (Gibco, catalogue no. 22400-089) supplemented with 10% FBS (Omega Scientific, catalogue no. FB-02). To establish tumours, 1âÃâ106âSNU16 cells in 200âµl of a 1:1 mixture of PBS and Matrigel (Corning, catalogue no. 354234) were given by subcutaneous injection into the right flank of 9-week-old female severe combined immunodeficient beige mice (Envigo, strain code 186). Tumour measurements were taken two to three times per week and body weights were taken daily. Tumour volume measurements were obtained using digital calipers and tumour volumes (mm3) were determined using the formula: tumour volumeâ=â(LâÃâW2)/2, where L is the length/largest tumour diameter and W is the width/shortest tumour diameter, with all tumours collected before reaching 1,500âmm3. Animals (eight mice per group, which historically allowed for significance determination between vehicle and infigratinib) were randomly assigned to unblinded treatment with vehicle, infigratinib (15âmgâkgâ1 oral (PO) once-daily (QD)), BBI-2779 (30âmgâkgâ1 PO every other day (Q2D)) or the combination of BBI-2779 and infigratinib once average tumour volume was 285â(±10)/mean (±s.e.m.)âmm3. One vehicle tumour was taken down on day 22; the mouse was sacrificed due to large tumour volume. Infigratinib was formulated in a 1:1 mixture of sodium acetate buffer, pH 4.6 and polyethylene glycol 300. BBI-2779 was formulated in 0.5% methylcellulose (Sigma-Aldrich, catalogue no. M0512) and 0.2% Tween 80 (AG Scientific, catalogue no. T-2835) in HyPure Molecular Biology Grade Water (HyClone, catalogue no. SH30538.02). Dose holidays were provided to individual animals that demonstrated greater than â10% body-weight change from baseline, and Nutra-Gel was provided to the entire treatment group. Animals were sacrificed 6âh, 24âh or 36âh after the last dose, and tumours were collected for western blot or copy number analysis.
Copy number analysis from xenograft samples
For copy number analysis, tumours were cut into 10â20âmg pieces and flash-frozen in liquid nitrogen. DNA was extracted using the QIAcube DNA Extraction Kit (Qiagen, no. 51331). Briefly, a mixture of buffer ATL and proteinase K was added to the frozen tumour pieces, and they were set out to equilibrate to room temperature. Tumours were then vortexed for 30âs and placed into an incubator at 56â°C to digest overnight. The next morning, an additional 150âμl of buffer ATL was added and samples vortexed for an additional 30âs to reduce the viscosity of the samples before transfer to the S block. Qiagen protocol for the 96 QIAcube HT was followed for the remainder of the DNA isolation. Purified DNA was quantified for the presence of double-stranded DNA on the QIAxpert (Qiagen, catalogue no. 9002340). The DNA was diluted to 5ângâµlâ1 (5à working stock) in RNase/DNase free water (Thermo Fisher Scientific, catalogue no. 10977015) and 2âµl was loaded into a 384-well plate. Master mix recipe (Master Mix (2Ã), 5.5âµl; CNA (Target Gene) 20Ã, 0.55âµl; CNR telomerase reverse transcriptase (TERT) 20Ã, 0.55âµl; nuclease-free water, 2.2âµl) was made containing TaqPath Pro Master Mix 2à (Thermo Fisher Scientific, catalogue no. A30866) human female genomic DNA (Promega, catalogue no. G1521) as a reference, internal controls (human TERT) and FGFR2 or MYC target gene probe (Thermo Fisher Scientific, catalogue no. 4400292). Reactions were run on the QuantStudio 6 or 7 (Thermo Fisher Scientific) using the qPCR reaction settings as follows: denature/enzyme activation: 95â°C, 10âmin; 40 cycles of denature 95â°C, 15âs; anneal/extend 60â°C, 60âs.
Quantifications and statistical analysis
All statistical methods and sample size have been stated in figure legends or the Methods section. No statistical methods were used to predetermine the sample size. The default test type was a two-sided statistic test, unless indicated in the text. The investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.