Culture and maintenance of undifferentiated human stem cells
The hESC line H9 (WA09-WiCell, RRID:CVCL_9773), along with its reporter-expressing derivatives (hSYN-ChR2–eYFP and NOS1-GFP), and the induced PSC line WTC-11 (UCSFi001-A, RRID:CVCL_Y803), were authenticated and karyotyped by the respective commercial providers. hPSCs were cultured on geltrex-coated plates and maintained in chemically defined medium (E8) as described previously16. The cultures were tested for mycoplasma every 30 days.
ENCC induction
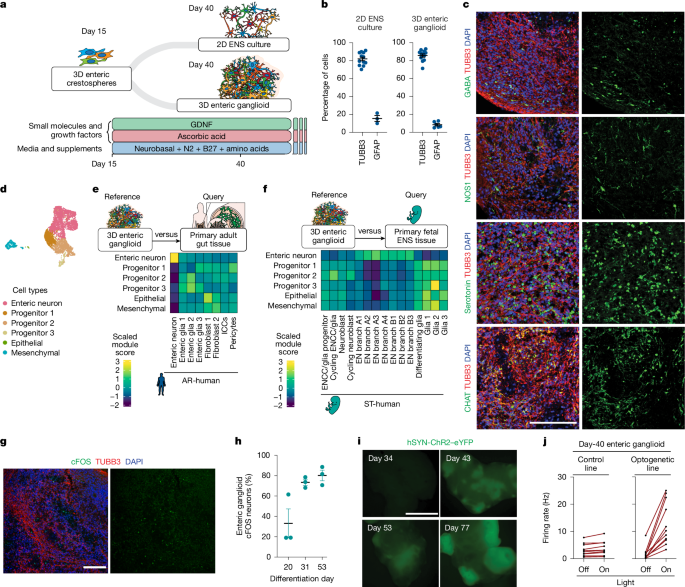
When the monolayer cultures of hPSCs reached about 70% confluency, a previously established 12-day ENCC induction protocol was initiated16,17 (day 0) by aspirating the maintenance medium (E8) and replacing it with NC induction medium A (BMP4 (1 ng ml−1), SB431542 (10 μM) and CHIR 99021 (600 nM)) in Essential 6 medium). Subsequently, on ENCC induction days 2 and 4, neural crest induction medium B (SB431542 (10 μM) and CHIR 99021 (1.5 μM) in Essential 6 medium), and on days 6, 8 and 10, medium C (medium B with retinoic acid (1 μM)) were fed to the cultures. Next, ENCC crestospheres were formed during day 12–day 15 to facilitate the selection for ENCC lineage and against contaminating ones in our cultures. In doing so, we removed ENCC induction medium C on day 12 and detached the ENCC monolayers using Accutase (30 min, 37 °C, 5% CO2). After centrifuging the samples at 290g for 1 min, we resuspended the ENCCs in NC-C medium (FGF2 (10 ng ml−1), CHIR 99021 (3 μM), N2 supplement (10 μl ml−1), B27 supplement (20 μl ml−1), glutagro (10 μl ml−1) and Minimum Essential Medium non-essential amino acids (MEM NEAAs; 10 μl ml−1) in neurobasal medium) and transferred them to ultralow-attachment plates to form free-floating 3D enteric crestospheres. On day 14, when the free-floating enteric crestospheres could be observed, we gently gathered them in the centre of each well using a swirling motion. Then, the old medium was carefully aspirated from the circumference of each well without removing the crestospheres. After addition of the fresh NC-C medium, the cultures were incubated for 24 h (37 °C and 5% CO2) before the enteric neuron induction phase.
Enteric neuron induction from enteric neural crests
On day 15, enteric crestospheres were gathered in the centre of the wells using a swirling motion and NC-C medium was removed using a P1000 micropipette in a slow circular motion, avoiding the free-floating crestospheres. At this step, the protocol varied depending on the final desired culture layout (2D ENS cultures versus 3D enteric ganglioids). For 2D ENS cultures, after washing the enteric crestospheres with PBS, Accutase (Stemcell Technologies, 07920) was added, and plates were incubated for 30 min at 37 °C to dissociate the crestospheres. Then, remaining spheroids were broken by pipetting enteric neuron medium (GDNF (10 ng ml−1), ascorbic acid (100 μM), N2 supplement (10 μl ml−1), B27 supplement (20 μl ml−1), glutagro (10 μl ml−1) and MEM NEAAs (10 μl ml−1) in neurobasal medium). Cells were spun (2 min, 290g, 20–25 °C), and supernatant was removed. Pellet was resuspended in enteric neuron medium, and cells were plated on plates coated with poly-l-ornithine, laminin and fibronectin at 100,000 viable cells per square centimetre. For 3D enteric ganglioids, we avoided Accutase treatment, and enteric crestospheres were fed with the same volume of enteric neuron medium (GDNF (10 ng ml−1), ascorbic acid (100 μM), N2 supplement (10 μl ml−1), B27 supplement (20 μl ml−1), glutagro (10 μl ml−1) and MEM NEAAs (10 μl ml−1) in neurobasal medium). Feeding continued every other day with enteric neuron medium until day 30–day 40, after which, feeding frequency could be reduced to once or twice per week but with a larger volume of feeding medium.
Immunofluorescence
For immunofluorescence staining, cells were initially fixed in 4% PFA in PBS (30 min, room temperature) and then blocked and permeabilized by permeabilization buffer (Foxp3/Transcription Factor Staining Buffer Set, 00-5523) for another 30 min at room temperature. After fixation and permeabilization steps, cells were incubated in primary antibody solution overnight at 4 °C, and then washed three times with permeabilization buffer before their incubation with fluorophore-conjugated secondary antibodies at room temperature. Before imaging, stained cells were incubated with DAPI fluorescent nuclear stain and washed an additional three times. The list of antibodies and working dilutions is provided in Supplementary Table 1.
Preparation of enteric ganglioid frozen sections
hPSC-derived ganglioids were collected, rinsed twice in PBS and fixed on ice in 4% PFA (SCBT sc-281692) for 3 h, followed by replacing 90% of the supernatant with PBS for storage at 4 °C for up to 6 months. Ganglioids were treated with 5% sucrose (RPI Research Products 524060) in PBS for 10 min at room temperature, followed by 10% sucrose in PBS for 2 h at room temperature and 20% sucrose at 4 °C overnight. Sucrose-treated ganglioids were positioned in cryomoulds (Tissue-Tek Cryomold medium, VWR 25608-924), all 20% sucrose was removed and they were incubated in 2:1 20% sucrose/OCT (Tissue Plus O.C.T. Compound Fisher HealthCare 5484) for 2 h at room temperature before flash freezing in ethanol–dry ice. Sections (of 12–20 μm) were taken on a cryostat (Leica 3050S), adhered to a Superfrost Plus Micro Slide, Premium (VWR 48311-703) and dried at 42 °C on a slide dryer for up to 2 h before storing at −80 °C for up to a year.
Staining enteric ganglioid frozen sections
Unless otherwise specified, all steps were performed at room temperature. Ganglioid frozen sections were prepared as above and then washed three times in PBS and blocked for 1–2 h in serum (10% donkey or 10% goat) with 0.5% (v/v) Triton X-100 (VWR 0694). Slides were then incubated with primary antibody diluted in serum (10% donkey or 10% goat) with 0.1% Triton X-100 at 4 °C for 12–20 h. Slides were washed six times for 20 min each in PBS with 0.1% Tween-20 (Sigma P1379) and incubated for 1 h with Alexa Fluor-conjugated secondary antibodies. The diluted secondary antibody solution was removed and replaced with 1.0 μg ml−1 DAPI in water for 10 min. The slides were washed six times for 20 min each in PBS with 0.1% Tween-20, and coverslips were mounted with Fluoromount-G (Southern Biotech 0100-01). The list of antibodies and working dilutions is provided in Supplementary Table 1. Images were acquired on a Leica SP8 inverted confocal or on the Echo Revolve. For images that were stitched, we used Leica’s LAS X tiling feature or the Grid/Pairwise stitching plugin for FIJI54.
Two-photon fluorescence imaging
Imaging experiments were conducted on a custom-built upright two-photon microscope operating with µManager software (San Francisco, CA). The excitation source was a two-photon Coherent Chameleon Vision II laser operating at 760 nm. Images were collected using an Olympus LWD 1.05-numerical-aperture water immersion objective (Olympus). An emission filter collecting light between 380 nm and 420nm (Chroma) was used to image DAPI, whereas the fluorescence emission of Alexa 568 was collected using a filter between 565 nm and 635 nm (Chroma).
Macro fluorescence imaging
Images were taken on a Nikon AZ100M ‘Macro’ laser scanning confocal instrument configured with long-working-distance low-magnification lenses. The microscope is equipped with the standard 405 nm, 488 nm, 561 nm and 640 nm laser lines and has photomultiplier tube detectors with a detection range from 400 nm to 700 nm. To reduce signal drop-off at the image edges, we used an optical zoom factor of ×2.1 and increased our lateral resolution using a digital zoom factor of ×1.873.
Flow cytometry
For preparation of samples for flow cytometry analysis, cells were initially dissociated into single-cell suspensions by Accutase treatment (Stemcell Technologies, 07920, 30–60 min, 37 °C, 5% CO2) and then fixed and permeabilized using fixation and permeabilization buffers (Foxp3/Transcription Factor Staining Buffer Set, 00-5523). Cells were stained with primary and secondary antibodies as described above for immunofluorescence. Flow cytometry was conducted using a BD LSRFortessa cell analyser and data were analysed using Flowjo (Software Version 8.7). The list of antibodies and working dilutions is provided in Supplementary Table 1.
hSYN-ChR2–eYFP enteric ganglioid blue-light activation
Enteric ganglioids were either exposed to blue light (100% laser intensity, 3 × 1-min exposure with 30-s intervals, EVOS FL) or left out in ambient light. Enteric ganglioids were then incubated for 45 min at 37 °C before dissociation, fixation and permeabilization for flow cytometry (see above). Cells were stained using antibodies to cFOS (Abcam, ab190289) and TUBB3 (Biolegend, 801202).
Bulk RNA-sequencing data analysis
Total RNA was extracted using the PureLink RNA Mini Kit. First-strand cDNA was then synthesized with the Quantseq Forward Library preparation kit from Lexogen. Illumina-compatible RNA-sequencing libraries were prepared with Quantseq and pooled and sequenced on an Illumina Hiseq 4000 platform at the University of California, San Francisco (UCSF) Center for Advanced Technology. Unique molecular identifiers were extracted from the fastq files with umi_tools, and cutadapt was used to remove short and low-quality reads. The reads were aligned to the human GENCODE v.34 reference genome using STAR aligner, and the duplicate reads were collapsed using umi_tools. Gene level counts were measured using HTSeq and compared using DESeq2.
Single-cell and single-nucleus RNA-sequencing sample preparation and data collection
All tubes and pipette tips used for cell collection were pretreated with 1% BSA in 1× PBS. Cells were dissociated in Accutase (Stem Cell) at 37 °C, in 10-min increments, with end-to-end rotation, until a single-cell suspension was obtained. The cells were washed in Cell Staining Buffer (Biolegend) and stained with TotalSeq HTO antibodies for 30 min on ice. The cells were washed twice in Cell Staining Buffer and filtered through a 40-µm pipette tip strainer (BelArt). The cells were counted using Trypan blue dye and a haemocytometer and pooled for sequencing. Single-cell RNA-sequencing libraries were prepared with Chromium Next GEM Single Cell 3′ Kit v3.1 (10x Genomics), with custom amplification of TotalSeq HTO sequences (Biolegend). The libraries were sequenced on an Illumina NovaSeq sequencer in the Center for Advanced Technologies (UCSF). The cell feature matrices were extracted using kallisto/bustools, and demultiplexed using seurat.
Quality control and cell filtration
Datasets were analysed in R v4.0.3 with Seurat v4 (ref. 55). The number of reads mapping to mitochondrial and ribosomal gene transcripts per cell was calculated using the PercentageFeatureSet function. Cells were identified as poor quality and subsequently removed independently for each dataset on the basis of the number of unique features captured per cell, the number of unique molecular identifiers captured per cell and the percentage of reads mapping to mitochondrial transcripts per cell. Dataset-specific quality control metric cutoffs can be found in Supplementary Table 2.
Dimensionality reduction, clustering and annotation
In cases in which it was applicable, biological replicate samples were first merged using the base R merge function. Count matrices were log-normalized with a scaling factor of 10,000, and 2,000 variable features were identified using the vst method. For datasets specified in Supplementary Table 3, count matrices of biological replicate samples were integrated using Seurat integration functions with default parameters. Cell cycle phase was predicted using the CellCycleScoring function with Seurat’s S and G2M features provided in cc.genes. The variable feature sets were scaled and centred, and the following variables were regressed out: nFeatures, nCounts, mitochondrial gene percentage, ribosomal gene percentage, S score and G2M score. Principal component analysis was run using default settings, and UMAP dimensionality reduction was performed using the principal component analysis reduction. The shared nearest neighbour (SNN) graph was computed using default settings, and cell clustering was performed using the default Louvain algorithm. Quality control metrics were visualized per cluster to identify and remove clusters of low-quality cells (less than average nFeatures or nCounts and higher than average mitochondrial and ribosomal gene percentage; Supplementary Table 3). The above pipeline was performed again on datasets after the removal of any low-quality cell clusters and for the subclustering analysis of the enteric neural crest, enteric neurons, nitrergic neurons and enteric glia. The number of principal components used for UMAP reduction and SNN calculation was determined by principal component standard deviation and varied for each dataset. The number of principal components used for SNN and UMAP calculation and the resolution used for clustering of each dataset are available in Supplementary Table 3. Cluster markers were found using the Wilcoxon rank sum test, and clusters were annotated on the basis of the expression of known cell-type marker genes (Supplementary Table 4). Following cell-type annotation, gene dropout values were imputed using adaptively thresholded low-rank approximation (ALRA)56. The rank-k approximation was automatically chosen for each dataset, and all other parameters were set as the default values. The imputed gene expression is shown in all plots and used in all downstream analysis unless otherwise specified.
Analysis of published datasets
Quality control
Criteria used by the original authors of each dataset were used to identify and remove poor-quality cells. Dataset-specific quality control metric cutoffs are provided in Supplementary Table 2.
Dimensionality reduction and clustering
Datasets were analysed with Seurat using the methods and parameters described by the original authors.
AR-human
For all datasets, count matrices were log-normalized with a scaling factor of 10,000, and 2,000 variable features were identified using the vst method. Batch correction by Unique_ID was performed using mutual nearest neighbours correction with the RunFastMNN Seurat Wrappers function. The dataset-specific parameters used for the RunUMAP, FindNeighbors and FindClusters functions are provided in Supplementary Table 3. Cell annotations determined by the authors were used for cell types and neuronal subtypes.
ST-human
Datasets downloaded from the Gut Cell Atlas (https://www.gutcellatlas.org/) retained the author’s original clustering annotations and dimensionality reduction coordinates.
For consistency of comparison, gene dropout values were imputed using adaptively thresholded low-rank approximation for all published datasets using automatically determined rank-k approximations and all other default values. The imputed gene expression is shown in all plots and used in all downstream analysis unless otherwise specified.
Cell-type transcriptional signature scoring
To find transcriptionally similar cell populations between two datasets, first the differentially expressed genes of the reference dataset were calculated from the non-imputed gene counts with the FindAllMarkers function using the Wilcoxon rank sum test, and only genes with a positive fold change were returned. The differentially expressed gene lists were first filtered to remove genes not present in the query dataset. Then for each cell cluster in the reference dataset, a transcriptional signature gene list was made from the top 100 differentially expressed genes sorted by increasing adjusted P value. The query dataset was then scored for the transcriptional signature gene lists of each reference dataset cell cluster using the AddModuleScore function on the basis of the query dataset’s imputed gene counts.
To identify nitrergic neurons, enteric neurons that expressed NOS1+ were scored for a module consisting of the rate-limiting synthesis enzyme(s), metabolism enzymes and transport proteins (NOS1, NOS1AP, ARG1, ARG2, ASL and ASS1) using the AddModuleScore function. A neuron was then annotated as nitrergic or NO if the cell’s expression of NOS1 was greater than 0 and the cell’s module score for the module above was greater than 0. A cell was annotated as other if both criteria were not met.
PP121 versus control gene expression correlation
To compare the gene expression of control and PP121-treated cell types, and neuronal subtypes and NO neuron subtypes, a subset dataset of each cell-type and subtype annotation was first created. For each subset, the non-imputed average expression of all genes was then calculated for the control and PP121-treated cells using the AverageExpression function and natural log-transformed for plotting. R2 values comparing the control and PP121 natural log expression values were calculated from linear modelling using the y ~ x formula, where y is modelled as a function of x.
cFOS expression screening
Day 90 enteric ganglioids were dissociated using Accutase, and single-cell suspensions (in ENC medium) were distributed in wells of V-bottom 96-well plates. Compounds from a neuronal signalling compound library (Selleckchem) were added at 1 μM using a pin tool, and cells were incubated for 75 min at 37 °C. Afterwards, cells were washed with PBS, and were immediately fixed for flow cytometry.
NO release assay
For high-throughput measures of NO release, day-40 2D ENS cultures (96-well plates) were used. After washing cells with Tyrode’s solution (NaCl (129 mM), KCl (5 mM), CaCl2 (2 mM), MgCl (1 mM), glucose (30 mM) and HEPES (25 mM) at pH 7.4), 70 μl of Tyrode’s solution was added to each well. Neuronal signalling compounds (Selleckchem) were added at 1 μM using a pin tool. After a 45-min incubation at 37 °C, supernatants were used to determine NO release using an NO assay kit (Invitrogen, EMSNO). In brief, the kit uses the enzyme nitrate reductase, which converts nitrate to nitrite that is then detected as a coloured azo dye absorbing light at 540 nm. NO release for each compound was presented as the A540nm relative to the vehicle (dimethylsulphoxide).
High-throughput screening to identify compounds that enrich NO neurons
Day-15 enteric crestospheres derived from H9 hESCs were dissociated into single cells (Accutase, Stemcell Technologies, 07920, 30 min, 37 °C), resuspended in enteric neuron medium and transferred into 384-well plates. Plates were incubated for 2 h for cells to attach. Using a pin tool, drugs from a library of 1,694 inhibitors (SelleckChem) were added to wells at the final concentration of 1 μM, and plates were incubated with drugs until day 20, when medium was changed to enteric neuron medium with no drugs. At day 40, cells were fixed, stained for NOS1 and imaged using an InCellAnalyzer 2000 (GE Healthcare). Hits were selected on the basis of the fold increase of the percentage of NOS1+ cells relative to the wells treated with vehicle (dimethylsulphoxide).
Drug–target interaction prediction
We obtained canonical SMILES of our hits from PubChem4,5) and generated a list of their known and predicted targets by combining data from the following databases: BindingDB (https://www.bindingdb.org/), Carlsbad (http://carlsbad.health.unm.edu/), DINIES (https://www.genome.jp/tools/dinies/), PubChem BioAssay (https://pubchem.ncbi.nlm.nih.gov/, filtered for active interactions), SEA (http://sea.bkslab.org/, filtered for MaxTC >0.4), SuperDRUG2 (http://cheminfo.charite.de/superdrug2/) and SwissTargetPrediction (http://www.swisstargetprediction.ch/).
In vivo cell transplantation
Specific pathogen-free homozygote neuronal nitric oxide synthase-knockout mice (B6.129S4-Nos1tm1Plh/J; nNos1−/−) were bred and maintained, in individually ventilated cages, for use as recipients. Animals used for these studies were maintained, and the experiments were performed, in accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by the University College London (UCL) Biological Services Ethical Review Process. Animal husbandry at UCL Biological Services was practised in accordance with the UK Home Office Certificate of Designation. This was practised according to the institute’s standard protocols, and no impact on the experimental outcomes was anticipated. As Nos1−/− mice are immunocompetent, cyclosporin A (250 μg ml−1 in drinking water) was administered orally 2 days before transplantation to reduce the possible rejection of donor human cells. Cyclosporin A-treated Nos1−/− mice from both sexes were chosen at random, from within littermate groups, and day-40–60 enteric ganglioids were transplanted to the wall of the distal colon of postnatal day 23–27 mice, through laparotomy under isoflurane anaesthetic. In brief, the distal colon was exposed and enteric ganglioids, containing 0.5–1 million cells, were subsequently transplanted to the serosal surface of the distal colon, by mouth pipette, using a pulled glass micropipette. Each transplanted tissue typically received three ganglioids, which were manipulated on the surface of the distal colon, with the bevel of a 30-G needle, to ensure appropriate positioning. Transplanted Nos1−/− mice were maintained with continued free access to cyclosporin A (250 μg ml−1)-treated drinking water for up to 8 weeks post-transplantation, to ensure extended immunosuppression, before euthanization and removal of the colon for analysis. The sample size for this study was determined on the basis of previous experience and known variability in these transplantation and engraftment assays. No statistical test was performed to determine the sample size. Cages and animals were randomly assigned to experimental groups to reduce potential bias. Blinding was not performed during transplantation or analysis. As cyclosporin A can affect several signalling pathways and induce gene expression changes, it is crucial to verify immunofluorescence results using appropriate controls such as tissue from cyclosporin A-treated untransplanted animals in follow-up studies. In addition, other immunocompromised backgrounds (for example, NSG) will be important to further verify these engraftment results.
Tissue preparation and fixation
Following the excision, the entire colon was pinned in a Sylgard (Dow)-lined Petri dish and opened along the mesenteric border. The mucosa was subsequently removed by sharp dissection, and tissues were fixed in 4% PFA in PBS (45 min–1 h, 22 °C) for further processing.
Tissue staining
Colonic longitudinal muscle myenteric plexus tissues were fixed with 4% PFA (1 h on ice), Thermo Scientific, J19943-K2) and blocked and permeabilized with a buffer containing 1% BSA and 1% Triton X-100 (in PBS, 45 min, room temperature). Then, tissues were incubated with primary antibody solutions (in the same buffer, overnight, 4 °C) and were washed three times before treatment with fluorophore-conjugated secondary antibodies (1 h, room temperature). Samples were stained with DAPI and washed before mounting using Vectashield (Vector Laboratories, H-1400). Antibodies are listed in Supplementary Table 1.
MEA analysis
Data acquisition
Neuron activity was recorded with the Axion Maestro Edge on Cytoview MEA 24-well plates in 1-h recording sessions for each condition. Neuormodulator or vehicle were added by removing the plate from the Maestro Edge, half-changing the medium with 2× concentrated neuromodulator or vehicle in pre-warmed medium, and immediately returning the plate to the Axion to resume recording. Optogenetic stimulation was performed with the Axion Lumos attachment, stimulating all wells of the plate with 488-nm light at 50% intensity, 1 s on, 4 s off, 30 times.
Data processing
Raw data were first spike-sorted with a modified version of SpikeInterface (https://github.com/SpikeInterface) using MountainSort to identify high-quality units by manually scoring on the basis of amplitude, waveform shape, firing rate and inter-spike interval contamination. For pharmacology experiments, neurons were matched between vehicle and neuromodulator recordings by examining all detected units on a specific electrode after spike scoring and identifying units with identical waveforms. Firing rates of these ‘paired’ units from all wells that received the treatment were compared across the control and neuromodulator conditions. Positive responders were units that had a firing rate change greater than +0.1 Hz; negative responders had a firing rate change less than −0.1 Hz; neutral responders had a firing rate change between −0.1 and +0.1 Hz. For optogenetic experiments, individual units were again extracted with SpikeInterface and manually scored. Recordings were separated into ‘on’ times when the LED was active and ‘off’ times when it was not. All units were compiled, and firing rates for each unit were compared during the on and off windows.
Calcium imaging
Day-5 enteric ganglioids were plated on plates coated with poly-l-ornithine, fibronectin and laminin to be compatible with a microscopy-based calcium imaging assay and cultured until day 50. For real-time calcium imaging, medium was removed, and cultures were washed once with Tyrode’s solution (Boston Bioproducts, BSS-355). Then cultures were incubated with Tyrode’s solution supplemented with 2.5 μg ml−1 Fluo-4 AM (1041F, Ion Biosciences) and 1:100 Pluronic F-127 (Ion Biosciences) for 30 min at 37 °C. Then, dye-containing solution was completely removed and replaced with pre-warmed Tyrode’s solution. Plates were transferred to an Echo Revolve microscope, and baseline videos were recorded. During recording, Tyrode’s buffer containing 2× concentrated neurotransmitters (for a final concentration of 100 M) was added and recording was continued for an extra 30 s. Snapshots before and after neurotransmitter addition were used for quantification using FIJI57. For analysis, fluorescence intensities were quantified in the same regions of interest defined using automatically identified particles using a globally applied threshold. Fluorescent intensity calculations included all pixels within the region of interest. The data were reported as normalized fluorescence intensity for each particle, calculated by dividing the intensity after addition by the baseline intensity for each particle.
Ex vivo whole-organ colonic motility assays
Preparation of solutions
Krebs buffer (NaCl (117 mM), KCl (4.7 mM), NaH2PO4 (1.2 mM), MgCl2 (1.5 mM), CaCl2.2H2O (2.5 mM), NaHCO3 (25 mM), glucose (11 mM), pH 7.4) was placed in a 37 °C water bath and aerated with 95% O2 and 5% CO2 (carbogen) gas mixture for at least 30 min before experiment onset. ‘Drug’ treatment solutions were freshly prepared by adding the drug compound into Krebs buffer before starting data acquisition. The solution with NOS1 inhibitor was prepared by adding l-NAME to the drug solutions making drug + l-NAME.
Tissue dissection
For each experimental replicate, a pair of 8-week-old wild-type C57BL6 mice (male) were placed in a sealed chamber and euthanized using CO2 asphyxiation followed by cervical dislocation. The lower GI tract (caecum and colon) was removed and immediately transferred to 37 °C carbogenated Krebs buffer, with the faecal matter still inside. Adipose tissue and mesentery were removed before placing the colons in the organ bath reservoir of GI motility monitor (GIMM) apparatus. GIMM had two reservoirs making simultaneous acquisition of control and drug-treated colons possible.
Experimental set-up and procedure
GIMM was designed on the basis of a previously reported model58. The organ reservoir of GIMM has two chambers for recording two specimens simultaneously. It is connected to working solutions kept at 37 °C via a four-channel peristaltic pump (WPI, PERIPRO-4LS). Lower GI tract was collected and transferred to the organ bath with Krebs buffer flowing through. The caecum was pinned down at the proximal tip, and the distal end of the colon was pinned through the serosa and/or mesentery. Five 10-min (for the initial drug testing) or sequential 6-min (for the sequential drug treatment in the presence and absence of l-NAME) videos were recorded using the IC capture software (Imaging Source) with a high-resolution monochromatic firewire industrial camera (Imaging Source, DMK41AF02) connected to a 16-mm f/1.4 C-Mount Fixed Focal Lens (Fujinon HF16SA1). Tissue in the control chamber was exposed only to Krebs solution; the order of solutions in the experimental chamber was: Krebs, drug compound, Krebs (6 min each), l-NAME (2 min), l-NAME in the presence of a drug compound (6 min) and Krebs (6 min). The chambers were cleaned after each acquisition. Cages and animals were randomly assigned to experimental groups to reduce potential bias. Investigators were blinded during data acquisition but not blinded during data analysis.
Data and statistical analysis
VolumetryG9a was used to generate the spatiotemporal map of each acquisition59. Slow-wave and CMMC data were generated from spatiotemporal maps. Statistical analyses were performed using PRISM. The Kolmogorov–Smirnov test was used to compare cumulative frequency distributions in the CMMC and slow-wave analyses. For LCE count analysis, we performed one-way ANOVA.
GI transit analysis in mice
Mice were fasted for a period of 1 h before administration of 100 μl Brilliant Blue FCT (E133) solution (Cambridge Bioscience) to the stomach, by gavage, at 8 weeks post-transplantation. Following gavage, mice were returned to individual cages, with free access to food and water, and monitored continuously for visualization of dye in the stool. Total GI transit time was calculated from time of administration to the first visualization of dye in the stool (minutes).
Contractility analysis of colonic tissue
Longitudinal colonic muscle strips were isolated, and the mucosa was removed by sharp dissection in oxygenated Krebs solution. Longitudinal strips of the tunica muscularis were mounted in tissue baths (10 ml, SI-MB4; World Precision Instruments) connected to force transducers (SI-KG20, World Precision Instruments), via suture, under an initial tension of 0.5 g. Tissues were maintained at 37 °C with perfusion of oxygenated Krebs solution. Following a 60-min equilibration period, activity was recorded using a Lab-Trax-4 data acquisition system (World Precision Instruments) in the absence and presence of NANC conditions (atropine, 1 μM; phentolamine hydrochloride, 1 μM; propranolol hydrochloride, 1 μM). Following a basal recording of 20–25 min, EFS was applied for 30 s as trains of electrical pulses (5 Hz; 40 V; 0.3-ms pulse duration) at 5-min intervals, via platinum electrode loops placed at each end of the muscle strip, using a MultiStim System (D330, World Precision Instruments). Three rounds of EFS were applied in each condition to ensure that reproducible responses were observed. The total recording time for individual muscle strips was 1 h 45 min. Data were collected, stored and analysed by computer using a data acquisition program (Labscribe V4, World Precision Instruments).
Generating figure schematics
We used Adobe Illustrator (version 25.4.1) to generate schematics for the figures. Microsoft Excel version 16.96.1 and Graphpod Prism version 10 were used to generate graphs, and statistical data analysis. R v4.0.3 with Seurat v4 was used for single-nucleus RNA-sequencing data analysis.
Use of hESCs
This study used established and commercially available hESC lines and was approved by the UCSF Human Gamete, Embryo, and Stem Cell Research Committee.
Ethical approval statement for the animal study
Animals used for these studies were maintained, and the experiments were performed, in accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by the UCL Biological Services Ethical Review Process. Animal husbandry at UCL Biological Services was in accordance with the UK Home Office Certificate of Designation. All procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.