Study scope
The geographical scope of the study was sub-Saharan Africa, defined as comprising ecoregions51 in the Afrotropic realm within continental Africa. We calculated energy flows for the 1,088 mammal and 1,955 bird species for which data were available, composing 98% of total African species excluding seabirds. Energy flows were calculated independently for each 8 km × 8 km grid cell, the scale at which biodiversity intactness data are available. The study area comprises ~317,000 cells. To assess change over time, energy flows were calculated twice for each cell: once based on estimated historical species abundances in the pre-industrial and pre-colonial Holocene (approximately 1700 CE), and once based on contemporary abundances, given human land use, according to the population changes estimated by the BII28. A visual overview of the methods is presented in Extended Data Fig. 5.
Historical species abundances
To determine which bird and mammal species were historically present in each 8 km × 8 km grid cell, we used historical IUCN/Birdlife range maps, adjusted for species habitat requirements following ref. 52. For each species, the initial IUCN/Birdlife range maps were divided into 1/12° grid cells, and grid cells were excluded from the species range when the cell’s historic, natural biome did not match the species’ habitat requirements, as documented by the IUCN. The 1/12° grid cells were then aggregated into 1/2° cells, and cells were included if they contained any available habitat. For a few small-range species, 1/2° habitat-adjusted maps eliminated all available range due to their exclusion from 1/12° cells, so unadjusted IUCN/Birdlife ranges were used. For the 11 large mammal species for which historical range maps are not available within the IUCN database, we adapted vector maps from other sources, following ref. 44, and then applied the gridded habitat filter detailed in ref. 52 (Supplementary Information).
To calculate historical species abundances, we used published median population density estimates for bird5 and mammal4 species. These were modelled as a function of trait, environmental, and phylogenetic predictors, using additive mixed-effect models and Bayesian inference, based on ~36,000 empirical records of bird and mammal population densities across 737 mammal and 1,853 bird species. Population density estimates for species lacking empirical data were extrapolated using the model. To estimate species abundances across sub-Saharan Africa, we used mean species population densities2. Mean densities were calculated using log-normal distributions based on published median densities and uncertainty intervals. Because population density distributions for most species are left-skewed, mean species population densities are higher than median values for species with wide confidence intervals. Given that ~75% of the global terrestrial surface is modified by humans to some extent53, the exclusion of non-natural population densities is not realistically possible, and is not necessarily desirable given that hominids have modified African species population densities for millions of years.
Contemporary species abundances from the BII
To estimate contemporary species abundances we multiplied historical abundances by the proportional intactness of each species in each 8 km × 8 km cell under modern land use. We used the intactness values for species under various land uses that are published in the BII for Africa dataset3. The BII employs a structured expert elicitation process to estimate and validate the proportional changes to species abundances under nine land uses: strict protected areas, near-natural lands, rangelands, intensive croplands, smallholder croplands, tree croplands, timber plantations, dense settlements and urban areas. The BII allocates each species into one of 17 bird and 76 mammal ‘response groups’ containing species that respond similarly to land use change. The average impact of each land use class on the abundance of species in each response group was calculated from ~30,000 individual estimates produced by 200 experts on African biodiversity. To map changes in abundance, each cell was assigned a land use class and intensity according to the land use classification outlined by ref. 28. Cells within protected areas and timber plantations were classified categorically, and cells within croplands, rangelands, and settlements were classified and then scaled along a land use intensity gradient. In cases where land use change benefited a species, the intactness of that species was greater than 1, and its abundance increased compared to the historical baseline.
Daily energy expenditure and food uptake
To calculate ecosystem energy flows, we first calculated the short-term equilibrium rate of food consumption for each species following ref. 2. For each species, daily energy expenditure was calculated from body mass using multi-species allometric equations34 (Extended Data Fig. 7; see Supplementary Table 1 for equations). Food consumption was calculated from energy expenditure based on published assimilation efficiency values for each food type and taxonomic group of birds and mammals (Supplementary Table 2). Where available, assimilation efficiency values were assigned at the family level; otherwise they were assigned at the order or class level. Values for the body mass of each species, and for the composition of food types within each species’ diet, were derived from the Elton Traits database for mammals54 and from the Avonet database for birds55. Energetic food intake was calculated in units of kJ m−2 year−1 and then averaged across cells.
Allocation of species into trophic guilds and functional groups
To understand how human land use has altered ecosystem trophic structure, we allocated species into trophic (that is, feeding) guilds. Each species was allocated to a single trophic guild, to shed light on how an ecosystem’s trophic structure, defined as the distribution of energy among guilds, varies between biomes and land uses. Species were allocated to guilds based on their taxonomic class, their diet (for example, omnivore, carnivore, nectarivore, folivore or frugivore), and their lifestyle (for example, arboreal or terrestrial). Data on diet and lifestyle were extracted from the Elton Traits database for mammals54 and from the Avonet database for birds55. Throughout the text, herbivore is used as an umbrella term to capture species eating any kind of plant matter, including foliage, seeds and nuts, nectar and fruit. The terms folivore, granivore, nectarivore and frugivore are used to refer to these groups independently. In addition, large and small terrestrial herbivores were split according to a published list of African large herbivores44 to better isolate how the distinctive vulnerability of large herbivores to human activity alters ecosystem trophic structure.
To understand how human land use has altered ecosystem function, we allocated species into 23 functional groups: 11 for birds and 12 for mammals. Species that perform multiple functions were allocated to multiple groups, so that the sum of energy flows through functional groups is greater than the total flow through the ecosystem’s birds and mammals. By contrast, the energy flows through guilds sum to the total energy flow through birds and mammals. We adapted a list of 11 bird functions from a published list of major avian ecosystem functions14. We added a function for aquatic carnivory and subdivided the invertivory function based on species lifestyle (for example, insessorial, aerial, terrestrial), as invertivory is performed by over half of all bird species. We sorted birds into functional groups based on their lifestyles and diets (see Extended Data Table 1 for sorting criteria for both birds and mammals). Unlike for birds, there is no single authoritative source on functions performed by mammals. After reviewing the literature we designated twelve mammal functions performed by large herbivores13,31, carnivores36,56, primates35, bats57, fossorial mammals46 and other small mammals58. We sorted mammals into functional groups based on their diet, body mass and lifestyle. For the grazing and browsing functions performed by large terrestrial herbivores we used published data on the leaf versus grass component of large herbivore diets44, and included large, terrestrial, herbivorous primates (Gorilla spp. and Theropithecus gelada) based on the expert knowledge of the authors. We additionally used published data on herd size44 to select herbivores that perform a nutrient dispersal function, as herd forming species have a distinctive effect on nutrient distribution within ecosystems13. We included in the megafauna impacts function those species that have unique ecological impacts because their large body size frees them from predation31. We determined the diet thresholds for each function iteratively, running the species allocation process multiple times and refining thresholds based on the authors’ expert knowledge. To clarify our results in the main text, we further aggregated our 23 preliminary functions into 10 aggregate functions, some of which are performed by both birds and mammals (Extended Data Table 1).
Comparison of energy flows across functions, biomes and land uses
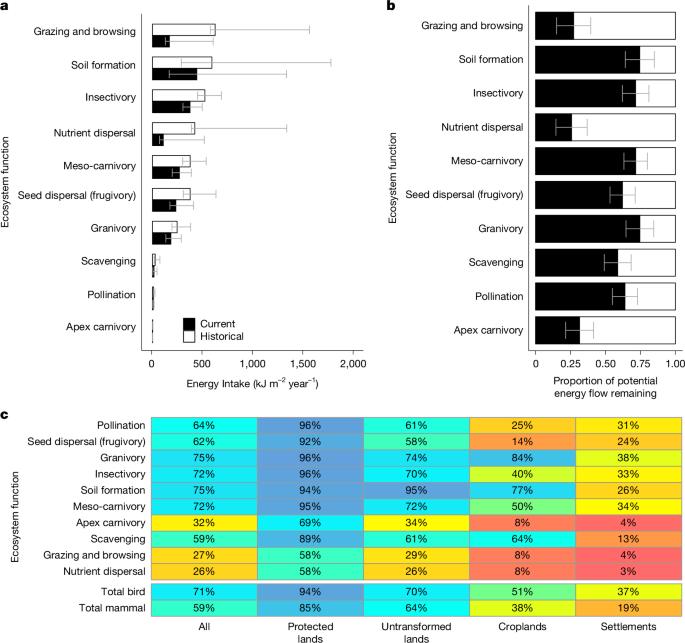
To calculate energy flows through functions, we summed the energy flows through all species that contribute to each function. This approach weights the contributions of species to associated functions based on species’ average daily energy consumption. The proportionate contribution of each species to its functions therefore changes depending on whether energy flows are calculated based on historical species abundances or based on present day, human impacted species abundances. Beyond energy flow, we did not scale species-level contributions to functions based on other metrics of functional efficiency: for example, based on pollen deposition rates, seed dispersal distance, or diet proportion. These causes of efficiency vary widely between functions and species14 and are difficult to measure consistently. To avoid biases, we therefore assumed that all species use energy equally efficiently to perform their associated ecosystem functions. For the analysis, we compared energy flow within specific functions across space and time. It is not meaningful to compare energy flows across ecosystem functions (for example, predation vs soil disturbance) as how each function uses energy is very different.
We also calculated the average energy flows through functional groups and trophic guilds across biomes and land uses. The biome is commonly proposed as the appropriate unit of analysis for assessing biodiversity trends, because biomes are biologically coherent subunits of the biosphere with structures and functions that respond to land use change in relatively consistent ways12. We allocated cells into biomes based on the biome map of the RESOLVE Ecoregions dataset51. To allow for broad comparisons between vegetation types, we further aggregated biomes into forests, grassy systems comprising savannas and grasslands, and arid systems comprising deserts and shrublands. For the biomes analysis, we excluded cells falling into the fynbos and thicket biomes, which are not easily classifiable and make up less than 2% of sub-Saharan Africa. We also excluded cells falling into mosaic biomes, as the low accuracy of available continent-scale vegetation maps makes it infeasible to subdivide mosaics into component biomes within the study scope. We calculated average energy flows through each guild and functional group across each of these three aggregated biomes under historical conditions and under modern land use conditions.
We allocated cells into land uses using an adapted version of the 8 km × 8 km resolution African land use map created for the BII for Africa28. Following source28, cells were allocated to four land uses: strict protected areas (IUCN categories I:III); settlements (>20% urban cover or a population density over 1,000 per km2); croplands (>20% crop cover); and unprotected untransformed land (remaining cells). We calculated average energy flows through each guild and functional group across each of these four land uses.
Comparison of energy flows to biodiversity intactness and species richness values
To understand how well biodiversity intactness values predict functional intactness, we related the BII of each cell to the intactness of energy flows through each cell. We related the BII of birds and mammal species to the intactness of total energy flows through bird and mammal species (Fig. 4a,b) and to the intactness of energy flows through species in each functional group (Extended Data Fig. 1). We identified functional groups for which biodiversity intactness is a good proxy (slope approximately 1 and high r2 value), a noisy proxy (slope approximately 1 and low r2 value), and a poor proxy (a non-linear slope or a slope that is not near 1). Functional groups with shallower slopes maintain high levels of energy consumption as biodiversity intactness declines, and were deemed more resilient to human impacts. We also related total energy flows to native bird and mammal species richness, to understand the extent to which high-energy keystone species versus rich communities of species drive ecosystem function (Fig. 4c,d). We analysed these relationships across all cells using linear regression.
Uncertainty calculation
Following ref. 2, we quantified uncertainty in our estimates of energetic intake by running 10,000 Monte Carlo simulations of energy flow through animal species and groups. For each simulation, we replaced the values in our original calculations with values drawn from random distributions. We assumed there was uncertainty in the following variables: species body mass, population density, daily energy expenditure equation (DEE), assimilation efficiency, and fractional diet composition of each species. Following ref. 3, we also assumed there was uncertainty in the estimated intactness of each species in each land use.
For body mass, we drew values from a truncated normal distribution (lower bound = 1 g) in which the mean was published mean body mass54,55 and standard deviation was 15% as described in ref. 2 For population densities, we drew from a log-normal distribution, using mean and uncertainty values for each species published in refs. 4,5. For DEE, we estimated the 95% confidence intervals following the methods described in ref. 34. For assimilation efficiency, we drew from a random beta distribution using the mean and standard deviation by taxonomic group and food type in the literature (Supplementary Table 1). For diet composition, we drew from a symmetrical beta distribution with uncertainty parameters assigned following ref. 2. For the proportional intactness of species abundances in each land use, we drew from a random beta distribution using the mean intactness values and standard deviations published in ref. 3. Intactness values were previously validated in ref. 3 through a structured expert elicitation process.
The uncertainty in each of these variables captures the natural variability occurring within species among individuals and groups, as well as ecologists’ uncertainty about mean values. For example, the population density of a given species will naturally vary geographically based on habitat suitability, resource availability, and competition. But there is also absolute uncertainty about the mean species population density of each species based on limitations on empirical data and model accuracy. This division of uncertainty into geographic and absolute components is true of the other variables as well. The uncertainty derived from natural variability decreases as there is an increasing number of analysed landscapes in which the species occurs. We assumed that half the uncertainty in species energy flow in a given landscape is from natural variability and that half is from absolute uncertainty about mean values, which does not decline as geographic area increases.
To account for the effects of area in our uncertainty estimates, we grouped species-level energy flows into 1° grid squares (~12,000 km2 at the equator) following ref. 44. We treated uncertainty about natural variability as independent in each 1° square in which a given species occurs and drew from independent distributions in each square. For each species, we calculated range-wide spatial means of energy flow for each of the 10,000 Monte Carlo simulations, and then propagated this area-scaled uncertainty into the absolute uncertainty about mean energy flow values generated from the area-independent Monte Carlo simulation estimates. We estimated total uncertainty by assuming uncertainty in all variables simultaneously, and calculated the 2.5th and 97.5th centiles intervals to derive 95% confidence intervals for our estimates.
Caveats
There are a number of caveats to our analysis, which we present here systematically in the order of their associated dataset or analysis. To estimate historical species abundances, we use range-wide average population densities for each species. For the vast majority of 3,000 bird and mammal species we analysed, there are insufficient data to predict how population densities vary along environmental gradients. We assumed that using flat densities would not substantially alter our results, hypothesizing that the intra-species variation in population densities would even out when summing energy flows across tens, hundreds, or thousands of species. To test these assumptions, we calculated the declines in energy flows through the 92 large herbivore species for which spatially variable population densities are available over the whole of sub-Saharan Africa44. Using flat instead of variable densities changed the total energy flow through all 92 species by less than 1%, justifying our assumption (Supplementary Discussion). Another issue with our flat densities is that they do not account for intra-specific competition. It is expected that species reach higher densities when competitors are missing. The approach may therefore overestimate energy flows through species-rich guilds in species-rich cells, although this was not supported in our sensitivity test.
A second set of caveats regards our use of range maps. Because these range maps are coarse (0.5° grid cells), they can overestimate abundances of species restricted to specialist habitats. Energy flows through colonial species including some fossorial rodents and water birds may be overstated. Another challenge is that the accuracy of the range map polygons varies between species groups and subregions, with maps of well-known species and well-known regions better accounting for fine-scale habitat heterogeneity. As a result, both historical energy flows and declines may be overestimated in poorly known areas, where maps are likelier to include inappropriate habitat not occupied by species. By contrast, where maps for poorly known species do not include historical ranges, declines in energy flows may be underestimated.
A third set of caveats regards our data on species traits and allometric equations. Our analysis also assumes that species’ diets, body masses and assimilation efficiencies do not vary consistently across land uses and biomes. However, a prior analysis of bird and mammal energetics across a land use gradient showed that shifts in diet had negligible effects on total energy flows. In addition, we accounted for substantial uncertainties around all three of these variables in our uncertainty analysis. A similar caveat is that the allometric equations, which we used to predict energy requirements based on species body sizes, do not account for environmental variables, for example the impact of temperature on energy needs. Consequently, the analysis may underestimate energy flows through cold regions, particularly afro-alpine and afro-temperate ecoregions. However, these regions make up a very small part of sub-Saharan Africa, which is overwhelmingly tropical or subtropical.
A fourth set of caveats regards the Biodiversity Intactness Index, used to estimate species responses to land use change. Because the BII averages responses from experts in different countries and regions, it does not account for how national political factors impact species abundances. These factors include war, protected area management, wildlife legislation, and cultural differences about hunting. The analysis may therefore overestimate energy flows in regions where unique national factors cause anomalously high overexploitation of wildlife, independent of land use transformation (and the converse where regions have anomalously low exploitation of wildlife such as taboos against bushmeat). In addition, only protected areas within IUCN categories I-III were designated as protected areas on the BII map. The analysis may therefore have overestimated energy intactness across the region’s de jure protected areas and underestimated intactness in some de facto strictly protected areas, for example private reserves. This study analyses continent-wide average energy flows through guilds in different land use classes and biomes, which are less likely to be affected by national factors. However, an effort to use this approach to analyse energy flows over smaller areas (for example, within a country or protected area) would need to account for regional and national variables affecting species abundances.
Finally, a fifth set of caveats regards species’ allocation into functional groups and the estimation of functional intactness. The study assumed that species contribute evenly to an ecosystem function, accounting for population density and allometry. For many functions, species were allocated based on their diets, after reaching a certain diet threshold (for example, 25% or 50%). The analysis may therefore overestimate absolute energy flows through functions performed by species that have a broader array of diets. However, this should not substantially affect comparisons of energy intactness within functions across time and space, the core aim of the study. In addition, for the vast majority of species and functions, there were insufficient data to estimate how variations in behaviour moderates the efficiency with which species use energy when performing functions. This caveat is less important for diet-based functions such as grazing, browsing, insectivory, granivore, and carnivory, where energy consumption by definition correlates closely with functionality. However the lack of data about behaviour may create more uncertainty for functions dependent on movement, such as seed dispersal, nutrient dispersal, and soil disturbance. The analysis may underestimate declines in these movement-dependent functions where species movements are highly constrained by habitat fragmentation (that is, in forests), even when landscapes and abundances remain relatively intact.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.