Chemicals and materials
Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O, 99.9%, ABCR), manganese(II) nitrate hexahydrate (Mn(NO3)2·6H2O, 98%, ABCR), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, 98%, ABCR), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, 98+%, ABCR), copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 98+%, Sigma), ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24 ·4H2O, 99%, Roth), indium(III) nitrate hydrate (In(NO3)3·H2O, 99.9%, Sigma), tin(II) chloride (SnCl2, 98%, Sigma), citric acid (99.5%, Acros), ethylene glycol (99+%, Brunschwig), cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O, 99.5%, ABCR), samarium(III) nitrate hexahydrate (Sm(NO3)3·6H2O, 99.9%, ABCR), ethylenediaminetetraacetic acid (99%, IVALUA), ammonia solution (25%, VWR), lanthanum(III) nitrate hexahydrate (La(NO3)3·6H2O, 99.9%, ABCR), strontium nitrate (Sr(NO3)2, 99+%, Fisher), LSGM (99%, Sigma), copper(II) oxide (CuO, 99%, Sigma), dispersant (2%, Fiaxell SOFC Technologies), binder (30%, Fiaxell SOFC Technologies) and carbon dioxide (CO2, 99.9%, Carbagas) were all used as received without further purification.
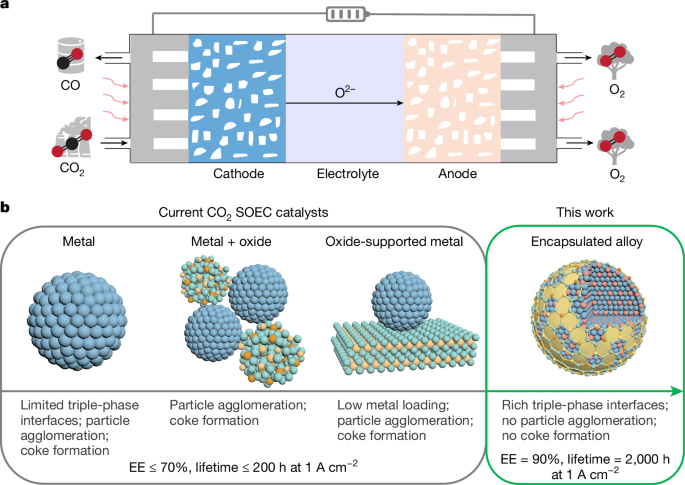
Synthesis of MxNi1−x@SDC
MxNi1−x@SDC was synthesized using a sol–gel method. First, 20 mmol of Ni(NO3)2·6H2O and 20 mmol of a second metal salt, which included Mn(NO3)2·6H2O, Fe(NO3)3·9H2O, Co(NO3)2·6H2O, Cu(NO3)2·3H2O, Zn(NO3)2·6H2O, (NH4)6Mo7O24·4H2O, In(NO3)3·H2O or SnCl2, were dissolved in 50 ml of H2O at 100 °C under vigorous stirring. Then, 100 mmol of citric acid and 100 mmol ethylene glycol were added to the solution to form a sol. Subsequently, 8 mmol of Ce(NO3)3·6H2O and 2 mmol of Sm(NO3)3·6H2O were added. The reaction was kept at 100 °C for about 3 h to evaporate H2O from the system. The remaining solvent was removed at 300 °C overnight in an oven, followed by heat treatment in air at 600 °C for 5 h with a ramping rate of 5 °C min−1. By changing the feeding molar ratio of Ni(NO3)2·6H2O and Co(NO3)2·6H2O while keeping their total amounts to 40 mmol, we obtained CoxNi1−x@SDC catalysts with different molar ratios of Co and Ni. A pure SDC sample without addition of Ni(NO3)2·6H2O or a second metal salt was also prepared.
Synthesis of Co0.5Ni0.5–SDC
Co0.5Ni0.5–SDC without an encapsulated structure was synthesized using a step-wise sol–gel method. First, pure SDC powder was first synthesized as described. Second, 10 mmol of SDC powder was dispersed in 50 ml of H2O at 100 °C under vigorous stirring, and 20 mmol of Ni(NO3)2·6H2O and 20 mmol of Co(NO3)2·6H2O were then added to the solution. Subsequently, 80 mmol of citric acid and 80 mmol ethylene glycol were added to form a sol. The reaction was kept at 100 °C for about 3 h to evaporate H2O from the system. The remaining solvent was removed at 300 °C overnight in an oven, followed by heat treatment in air at 600 °C for 5 h with a ramping rate of 5 °C min−1.
Synthesis of LSCF
LSCF was synthesized using a modified sol–gel method33. First, 40 mmol of ethylenediaminetetraacetic acid was added to 40 ml of a 25 wt.% aqueous ammonia solution. Subsequently, 12 mmol of La(NO3)3·6H2O, 8 mmol of Sr(NO3)2, 4 mmol of Co(NO3)2·6H2O and 16 mmol of Fe(NO3)3·9H2O were added. Next, 60 mmol of citric acid was added, and the solution pH was adjusted to 8 by addition of more aqueous ammonia solution. The resulting solution was heated at 100 °C under vigorous stirring for about 3 h to evaporate H2O from the system. The remaining solvent was removed at 120 °C overnight, followed by heat treatment in air at 950 °C for 5 h with a ramping rate of 10 °C min−1.
Cell preparation
An electrolyte-supported cell (MxNi1−x@SDC || SDC || LSGM || SDC || LSCF) was fabricated by a tape-casting process followed by several screen-printing processes. To prepare tape-casting slurries of the electrolyte, we mixed 20 g of LSGM powders, 14 g of dispersants and 20 g of binders by ball milling for 1 h. The resultant slurries were tape-casted into well-defined tapes with an original thickness of 1.5 mm using a tape-casting machine (MTI Corporation), followed by drying at room temperature for 48 h. The tapes were then punched into shape and sintered at 1,400 °C for 10 h with a ramping rate of 2 °C min−1 to form a dense electrolyte support. The screen-printing slurries were prepared by mixing 2 g of powders, 2 g of dispersants and 0.8 g of binders by ball milling for 1 h. A buffer SDC layer (2 wt.% CuO was added to promote sintering34) was first screen-printed on to both sides of the electrolyte support using a screen-printing machine (Fiaxell SOFC Technologies) to avoid any side reaction between electrolyte and electrode. Then, the MxNi1−x@SDC cathode and LSCF anode (35 wt.% SDC was added to the LSCF to improve O2− conductivity) were screen-printed on to either side of the SDC. The active areas of both cathode and anode were 1.5 cm2. The printed cells were sintered at 1,200 °C for 6 h with a ramping rate of 2 °C min−1. The thickness and mass loading of each layer are shown in Supplementary Table 1.
Electrochemical measurements
All electrochemical measurements were conducted using a SOEC set-up from Fiaxell SOFC Technologies and were controlled by an Autolab potentiostat (PGSTAT302N) equipped with a 20-A booster. The cell was heated to 800 °C with a ramping rate of 5 °C min−1 under air. Next, 20 vol.% H2 diluted with N2 was fed to the cathode compartment to reduce the catalyst for 10 min. When the reduction process was complete, CO2 and air were fed to the cathode and anode compartments, respectively, at a flow rate of 50 ml min−1. The gas outlet from the cathode compartment was connected to an online gas chromatograph (SRI Instruments) for product analysis.
The Faradaic efficiency was calculated by equation (1):
$$\rmFE=\fracn\times F\times x\times f_\rmouti,$$
(1)
where FE is the Faradaic efficiency; n is the number of electrons exchanged to produce CO from CO2 (that is, 2); and F, x, fout and i are the Faraday constant (96,485 C mol−1), the molar concentration of CO measured by gas chromatography (mol/mol), the flow rate of the cathode gas outlet (mol s−1) and the current (A), respectively.
The energy efficiency was estimated by equation (2):5
$$\rmE\rmE=\frac\Delta H\times \rmFE(\rmelectricity\;\rmenergy+\rmheat\;\rmenergy)=\fracE_\Delta H\times \rmFE(E_\rmapp+E_\Delta S/\eta _\rmh),$$
(2)
where EE is the energy efficiency; and ∆H, E∆H, Eapp, E∆S and ηh are the enthalpy of formation, thermoneutral potential, applied cell voltage, potential based on heat energy and heating efficiency, respectively. For the reaction CO2 → CO + 0.5 O2 at 800 °C, E∆H and E∆S are 1.467, and 0.481 V, respectively. The ηh used for industrial applications is usually at least 90% (refs. 35,36); thus, a value of 90% was used in our estimation.
The CO single-pass yield was calculated by equation (3):
$$\rmC\rmO\;\rmsingle\;\rmpass\;\rmyield=x\times f_\rmout/f_\rmCO_2\,,$$
(3)
where fCO2 is the flow rate of inlet CO2 gas (mol s−1).
Operando EIS measurements were performed at 1.0 V for CO2 electroreduction and 0.9 V for CO electrooxidation at 800 °C. A perturbation amplitude of 10 mV was applied with frequencies ranging from 100 kHz to 0.01 Hz. The fitting of the EIS data and DRT analysis were conducted using RelaxIS 3 software (rhd instruments).
ECSA was measured using a double-layer capacitance (Cdl) method37, determined by means of EIS measurements using a symmetrical cell configuration (MxNi1−x@SDC || SDC || LSGM || SDC || MxNi1−x@SDC) at 800 °C under N2 atmospheres on both sides. To establish a baseline, a flat, pure electrolyte cell without catalysts (SDC || LSGM || SDC) was fabricated. EIS data were recorded at 0 V with a perturbation amplitude of 10 mV and frequencies ranging from 100 kHz to 0.1 Hz. The electrode capacitance (Cdl) was obtained as half the capacitance derived from fitting the EIS using a simplified Randles circuit (Supplementary Fig. 5). Consequently, the ECSA of an electrode could be calculated by equation (4):
$$\rmE\rmCSA=C_\rmdl\times S/C_\rmbase\,,$$
(4)
where Cdl represents the capacitance of a specific electrode, Cbase represents the capacitance of the baseline and S denotes the geometric area of the electrode.
Characterizations
Metal elemental analysis was conducted on a NexION 350D inductively coupled plasma mass spectrometer. X-ray diffraction patterns were recorded on a PANalytical Aeris diffractometer using Cu Kα radiation (40 kV, 15 mA). Scanning electron microscopy images were recorded on a Zeiss GeminiSEM 300, and the corresponding energy-dispersive X-ray spectroscopy mapping was performed at 16 kV. TEM measurements were carried out on an FEI Talos F200S at 200 kV. Spherical-aberration-corrected TEM measurements were performed on an FEI Titan Themis 60-300 instrument at 200 kV. Carbon elemental analysis was performed in an UNICUBE (Elementar) microelemental analyser by a combustion method.
XPS measurements were performed in an Omicron XPS system equipped with a pretreatment chamber, using aluminium Kα X-rays as the excitation source at 15 kV and 300 W. For quasi in situ XPS measurements of fresh samples, a reduction process was applied in 20 vol.% H2 at 800 °C for 10 min in the pretreatment chamber. Subsequently, samples were transferred to the vacuum chamber for XPS measurements without exposure to air. After stability tests, samples underwent no further treatment but were immediately sealed in an N2-protected bag.
X-ray absorption spectroscopy (XAS) measurements were performed at the 12B2 Taiwan beamline (SPring-8, Japan) of the National Synchrotron Radiation Research Center, operated at an 8.0 GeV storage ring with a constant current of approximately 99.5 mA. To prevent oxidation, all samples were sealed in an N2-protected bag before XAS measurements. Measurements at the Ni K-edge (8,333 eV) and Co K-edge (7,709 eV) were performed in total fluorescence yield mode using a Lytle detector. The scan ranges were 8,133–8,933 eV for the Ni K-edge and 7,509–8,309 eV for the Co K-edge. XAS data were processed using Athena software, with energy calibration performed on the basis of the first inflection points in the absorption K-edges of the Ni and Co foils, which were set to 8333.0 eV and 7709.0 eV, respectively. Standard data processing procedures were applied, including background subtraction and edge height normalization. The average oxidation states of Ni and Co were determined by simulation of the linear combinations of reference spectra from Ni and Co foils and their oxides, NiO and CoO, respectively. Extended X-ray absorption fine structure analysis was performed using Fourier transforms on k3-weighted oscillations in a k range from 3.0 to 11.0 Å−1.
Operando Raman spectroscopy measurements were performed using an inVia confocal Raman microscope (Renishaw) equipped with a 532 nm laser, coupled with a Linkam heat stage capable of controlling temperature, gas atmosphere and cell voltage. The sample was first reduced in 20 vol.% H2/N2 at 800 °C for 10 min. Then, the atmosphere was switched to CO2, and Raman spectra were recorded at cell voltages of 1.2, 1.4, 1.6 and 1.8 V at 800 °C.
DFT simulations
DFT simulations were conducted using the Vienna ab initio simulation package38,39. The Perdew–Becke–Ernzerhof40 functional, supplemented with the D3 dispersion correction41, was used to account for the van der Waals interactions. A Hubbard correction42 was applied using the Dudarev method43 to describe the f-electrons of Ce and Sm in SDC models, with a Ueff of 4.5 and 4.0 eV for Ce and Sm, respectively31,44,45. Core electrons were represented using projector augmented wave core potentials46,47, whereas valence electrons were described with plane waves at a kinetic cut-off energy between 450 and 700 eV. The Monkhorst–Pack method48 was used to generate a Γ-centred mesh with a reciprocal grid finer than 0.036 Å−1 for Brillouin zone sampling.
Spin polarization was included in all simulations involving Co, Ni and oxygen-defective SDC systems45. A kinetic cut-off energy of 450 eV was used for most simulations, except for lattice parameter optimizations, in which a higher cut-off of 700 eV was applied to avoid Pulay stress. Dipole corrections49 were included along the z axis, and a vacuum region of 15 Å was added between slabs. For Ni and Co–Ni alloys, we modelled the (111) and (001) facets as p(4 × 4) and p(2 × 2) slabs, respectively. Three different terminations were considered for the Co–Ni(001) surface. Metal slabs contained four atomic layers, with the two outermost layers allowed to relax, whereas the two bottommost layers were fixed to their bulk positions. For SDC, a p(3 × 3) slab of the CeO2(111) was modelled, incorporating Sm and oxygen vacancies. This slab consisted of nine atomic layers (three O–Ce–O trilayers), with the five outermost layers allowed to relax. The Ni–SDC and Co–Ni–SDC interfaces were represented by Ce3SmO7 clusters adsorbed on p(4 × 4) slabs of Ni(001) and Co–Ni(001) surfaces, respectively. Six different Ce3SmO7 aggregates were generated on the basis of CeO2(111) models, in which a Ce atom was replaced by Sm, and an oxygen vacancy was introduced. Various adsorption configurations of these clusters were then explored on the metallic surfaces, resulting in 18 and 24 different structures on Ni(001) and Co–Ni(001), respectively.
For the adsorption energy of CO2 and CO and the energy profiles for CO2 electroreduction, CO2 and CO molecules in the gas phase were used as thermodynamics references. Vibrational contributions to enthalpy and entropy were included in the Gibbs free energies calculations, along with rotational and translational contributions for gas-phase CO2 and CO. Gibbs free energies were computed at 800 °C and 1 atm of CO2, in accordance with the experimental reaction conditions. Transition states were located using the climbing image nudged elastic band method50. Numerical frequencies calculations, using a step size of ±0.015 Å, were performed to confirm the nature of the transition states. Vibrational contributions to enthalpy and entropy for the Gibbs free energies of transition states were computed using standard approximations: the ideal gas model, rigid rotor and harmonic oscillator. Only the reactants were considered for metals, whereas for oxidic systems, oxygen atoms were also included in the vibrational estimations owing to their lower molecular weight.