Materials
M. marburgensis DSM 2133 and M. thermolithotrophicus DSM 2095 were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ). Most chemicals were from Sigma-Aldrich. H4MPT, methenyl-H4MPT+ and F420 were isolated from the M. marburgensis cells as described previously34. Methylene-H4MPT was chemically prepared from H4MPT with formaldehyde as previously described35. F420-dependent methylene-H4MPT dehydrogenase (Mtd) from Archaeoglobus fulgidus was purified from recombinant Escherichia coli cells as reported previously18. CoM-S-S-CoB was synthesized as described previously4,19,36.
Cultivation methods
The standard medium for culture of M. marburgensis contains 6.8 g l−1 (50 mM) KH2PO4, 2.544 g l−1 (24 mM) Na2CO3, 2.12 g l−1 (40 mM) NH4Cl, 0.2 mM MgCl2·6H2O, 50 µM FeCl2·4H2O, 5 μM NiCl2·6H2O, 1 µM CoCl2·6H2O, 1 µM NaMoO4·2H2O and 0.09 g l−1 Titriplex I. A concentrated trace element solution containing 0.2 M MgCl2·6H2O, 50 mM FeCl2·4H2O, 5 mM NiCl2, 1 mM CoCl2·6H2O, 1 mM NaMoO4·2H2O and 90 g l−1 Titriplex I was prepared separately and adjusted to pH 6.7 by addition of NaOH. The trace element solution (0.1% v/v) was added to the medium. To prepare the nickel- or iron-limiting medium, NiCl2·6H2O and FeCl2·4H2O were omitted from the trace element solution, and each metal ion concentration in the medium was controlled by addition of 50 mM FeCl2·4H2O or 5 mM NiCl2·6H2O solution to the medium. A 0.2% water solution of resazurin sodium salt was finally added to the medium (final concentration 0.6 mg l−1).
We used a 360-ml glass fermenter for the cultivation of M. marburgensis under the controlled nickel concentrations21. For cultivation, 80% H2/20% CO2/0.2% H2S mixed gas was supplied by a glass sparger (400 ml min−1) without overpressure as described previously. For the H2/CO2 gas-limiting condition, the mixed gas flow rate was reduced by half. The temperature of the glass vessel was controlled at 65 °C by circulating water from a water bath. The medium was stirred with a plastic stirrer bar at about 300 r.p.m. For the continuous culture, medium was fed by a peristaltic pump with a controlled flow rate. The gas phase of the feed medium was kept under a slight overpressure of N2 (less than or equal to about +0.1 bar) to compensate for the outflow of the medium (Supplementary Fig. 2). The cells were collected by anaerobic centrifugation using a Beckman JA-25.50 at 13,000g for 15 min at 4 °C.
For cultivation of M. thermolithotrophicus the standard medium 141 (H2/CO2) from DSMZ with modifications was used37. This medium contains 0.34 g l−1 KCl, 4.00 g l−1 MgCl2·6H2O, 3.45 g l−1 MgSO4·7H2O, 0.25 g l−1 NH4Cl, 0.14 g l−1 CaCl2·2H2O 0.14 g l−1, K2HPO4, 18 g l−1 NaCl, 2 ml l−1 of 1 g l−1 Fe(NH4)2(SO4)2·6H2O, 1 g l−1 sodium acetate, 0.5 ml l−1 sodium resazurin (0.1% w/v), 5 g l−1 NaHCO3, 0.5 g l−1 l-cysteine HCl·H2O, modified Wolin’s mineral solution without nickel 10 ml l−1, and Wolin’s vitamin solution 10 ml. pH was adjusted to 6.8–7.0. Modified Wolin’s mineral solution without nickel contains: 1.5 g l−1 nitrilotriacetic acid, 3 g l−1 MgSO4·7H2O, 0.5 g l−1 MnSO4·H2O, 1 g l−1 NaCl, 0.1 g l−1 FeSO4·7H2O, 0.18 g l−1 CoSO4·7H2O, 0.1 g l−1 CaCl2·2H2O, 0.18 g l−1 ZnSO4·7H2O, 0.01 g l−1 CuSO4·5H2O, 0.02 g l−1 AlK(SO4)2·12H2O, 0.01 g l−1 H3BO3, 0.01 g l−1 Na2MoO4·2H2O, 0.3 mg l−1 Na2SeO3·5H2O, 0.4 mg l−1 Na2WO4·2H2O. Wolin’s vitamin solution contains: 2 mg l−1 biotin, 2 mg l−1 folic acid, 10 mg −1 pyridoxine hydrochloride, 5 mg l−1 thiamine HCl, 5 mg l−1 riboflavin, 5 mg l−1 nicotinic acid, 5 mg l−1 calcium d-(+)-pantothenate, 0.1 mg l−1 vitamin B12, 5 mg l−1 p-aminobenzoic acid, 5 mg l−1 (dl)-α-lipoic acid. For the nickel-sufficient culture, 1 ml of NiCl2·6H2O (5 mM) was added to 1 l of medium (final Ni2+ concentration = 5 μM). In the nickel-limited culture, 10 ml of NiCl2·6H2O (5 μM) was added (final Ni2+ concentration = 50 nM). M. thermolithotrophicus was cultivated in a 100-ml vial sealed with a rubber stopper containing 20 ml liquid medium under a gas phase of 80% H2/20% CO2 (with +0.5 bar overpressure) at 65 °C with shaking (150 r.p.m.). The gas phase was replaced with a fresh gas mixture every 12 h. Three successive transfers of 5% inoculum to the culture medium containing 5 μM or 50 nM nickel were made from the culture medium containing 5 μM nickel. Cells from the third nickel-sufficient (Ni2+ = 5 μM) and nickel-limited (Ni2+ = 50 nM) cultures each in triplicate were collected to be used for proteomic analysis.
Preparation of cell extract
All steps were performed anaerobically in an anaerobic chamber under 3–5% H2 in N2 (Coy Laboratories). The frozen or fresh M. marburgensis cells (3.5 g) were suspended in 10.5 ml 50 mM Tris/HCl pH 7.6 containing 2 mM dithiothreitol. The cell suspension was subjected to ultrasonication on ice/water for 2 min using a SONOPULS GM200 (Bandelin) with a 72D tip with 30% cycles 12 times, with 2-min breaks between sonication cycles. The supernatant was collected by centrifugation in a Sorvall WX Ultra centrifuge (Thermo Fisher Scientific) with a T-880 rotor at 41,000 r.p.m. for 30 min at 4 °C. The supernatant (cell extract) contained 150 mg protein (11 mg ml−1). For the enzyme assay shown in Fig. 2c, the small molecules were removed from the cell extract by three successive rounds of ultrafiltration (10-kDa cutoff) and dilution. This is referred to as the washed cell extract.
Enzyme assays
Hmd activity
Hmd activity was determined by recording the formation of methenyl-H4MPT+ at A336nm by dehydrogenation of methylene-H4MPT under N2 (refs. 35,38). For the dehydrogenation assay, 0.68 ml of 120 mM potassium phosphate buffer pH 6.0 containing 1 mM EDTA was preincubated in a 1-ml quartz cuvette (1-cm light path) at 40 °C for 5 min. Typically, 10 µl of 1.4 mM methylene-H4MPT was added as the substrate to the cuvette to give a 20 µM final concentration. The enzyme reaction was started by addition of 10 µl of (typically 50-fold) diluted cell extract at 40 °C. The activity was calculated using the extinction coefficient of methenyl-H4MPT+ at 336 nm (21.6 mM−1 cm−1)39. One unit of the enzyme activity is defined as the formation or consumption of 1 µmol of methenyl-H4MPT+ per minute.
Mtd activity
Mtd activity was determined by recording the formation of methenyl-H4MPT+ at A336nm by dehydrogenation of methylene-H4MPT in the presence of F420 under N2 (ref. 21), in which the Hmd activity was fully inhibited by addition of an Hmd-specific inhibitor, TosMIC. For the assay, 0.66 ml of 120 mM potassium phosphate buffer pH 6.0 containing 1 mM EDTA was preincubated at 40 °C for 5 min. Typically, 7 µl of 100 µM TosMIC, 10 µl of 1.4 mM methylene-H4MPT and 10 µl of 1.4 mM F420 were added as substrate to the 1-ml quartz cuvette (1-cm light path) to give 20 µM final concentration each of methylene-H4MPT and F420. The enzyme reaction was started by addition of 10 µl of (typically 50-fold) diluted cell extract at 40 °C. The activity was calculated using the extinction coefficient of methenyl-H4MPT+ at 336 nm (21.6 mM−1 cm−1). One unit of the enzyme activity is defined as dehydrogenation of 1 µmol of methylene-H4MPT per minute.
Frh activity
Frh activity was determined by recording the reduction of F420 at A401nm under H2 (+0.4 bar)21. For the assay, 0.67 ml of 50 mM Tris/HCl pH 7.6 containing 10 mM dithiothreitol was preincubated at 55 °C for 5 min. Typically, 9 µl of 1.4 mM F420 was added as substrate to a 1-ml quartz cuvette (1-cm light path) to give 18 µM final concentration, and then 10 µl of 3.5 mM sodium dithionite was added to give a 50 µM final concentration. The enzyme activity was started by addition of 10 µl of (typically 20-fold) diluted cell extract at 55 °C. For dilution of the cell extract for the Frh assay, we used 50 mM Tris/HCl pH 7.6 containing 25 µM FAD. The activity was calculated using the extinction coefficient of F420 at 401 nm (25.9 mM−1 cm−1)39. One unit of enzyme activity is defined as reduction of 1 µmol of F420 per minute.
Mvh activity
Mvh activity was determined by recording the reduction of methyl viologen (MV) under H2 (+0.4 bar)21. For the assay, 0.67 ml of 50 mM Tris/HCl pH 7.6 containing 2 mM dithiothreitol was preincubated at 65 °C for 5 min. Typically, 7 µl of 200 mM MV was added as the substrate to a 1-ml quartz cuvette (1-cm light path) to give a 2 mM final concentration, and then 10 µl of 20 mM sodium dithionite was added to give a 290 µM final concentration to ensure the anaerobic condition. By addition of 10 µl of (typically 100-fold) diluted cell extract, the enzyme reaction was started at 65 °C. The activity was calculated using the extinction coefficient of MV at 604 nm (13.7 mM−1 cm−1)21. One unit of enzyme activity is defined as reduction of 2 µmol of MV per minute.
Benzyl-viologen-dependent Hdr activity
Benzyl-viologen-dependent Hdr (BV Hdr) activity was determined by recording the oxidation of reduced BV by heterodisulfide (CoM-S-S-CoB)3. For the assay, 0.7 ml of 800 mM potassium phosphate buffer pH 7.0 was preincubated at 65 °C for 5 min. A 7 µl volume of 200 mM BV was added to a 1-ml quartz cuvette (1-cm light path) to give a 2 mM final concentration, and then 10 µl of 20 mM sodium dithionite was added. A 10 µl volume of cell extract was added to the vial. The enzyme reaction was started by addition of 7 µl of 100 mM CoM-S-S-CoB to give a 1 mM final concentration at 65 °C. The activity was calculated using the extinction coefficient of BV at 578 nm (8.6 mM−1 cm−1)21. One unit of enzyme activity is defined as oxidation of 2 µmol of BV per minute.
H2-dependent Hdr activity
H2:CoM-S-S-CoB oxidoreductase activity was determined by monitoring the formation of thiols (CoM-SH and CoB-SH) from CoM-S-S-CoB using H2 as reductant3. For the assay, 1.0 ml of 1.6 M potassium phosphate buffer pH 7.0 was preincubated in a 5-ml amber vial at 65 °C for 5 min under H2 (+0.2 bar). A 50 µl volume of cell extract was added to the vial. The enzyme reaction was started by addition of 7 µl of 100 mM CoM-S-S-CoB (1 mM final concentration). A 100 µl aliquot of the reacted sample was diluted with 900 µl of 100 mM sodium phosphate buffer pH 8.0, to which 18 µl of 4 mg ml−1 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman reagent) was added and incubated at 25 °C for 10 min. The formation of thiol was calculated from the extinction coefficient of the Ellman reagent at 412 nm (14 mM−1 cm−1)18. One unit of enzyme activity is defined as the formation of 2 µmol of thiol per minute.
F420H2-dependent Hdr activity
F420H2:CoM-S-S-CoB oxidoreductase (F420 Hdr) activity was determined by monitoring the formation of thiols (CoM-SH and CoB-SH) from CoM-S-S-CoB using F420H2 formed by the Hmd+Mtd coupled reaction with H2 as the reductant in the assay. For the assay, 1.0 ml of 800 mM potassium phosphate buffer pH 7.0 containing 20 μM methenyl-H4MPT+ and 20 µM F420 was preincubated in a 1-ml quartz cuvette (1-cm light path) at 65 °C for 5 min under H2. After addition of 10 µl of 5 U ml−1 Hmd from M. marburgensis35 and 10 μl of 5 U ml−1 Mtd from A. fulgidus18, the assay solution was incubated at 65 °C for 5 min. Conversion of F420 to F420H2 was confirmed by monitoring absorbance at 401 nm, and then 7 µl of 100 mM CoM-S-S-CoB solution was added to the solution (1 mM final concentration). The enzyme reaction was started by addition of 50 µl of enzyme solution. A 100-µl aliquot of the reacted sample was diluted with 900 µl of 100 mM sodium phosphate buffer pH 8.0, to which 18 µl of 4 mg ml−1 Ellman reagent was added and incubated at 25 °C for 10 min. The formation of thiols was calculated from the extinction coefficient of the Ellman reagent at 412 nm (14 mM−1 cm−1). One unit of enzyme activity is defined as the formation of 2 µmol thiol per minute.
Fdh activity
Fdh activity was determined by monitoring the reduction of BV in the presence of sodium formate18. For the assay, 0.6 ml of 50 mM potassium phosphate buffer pH 7.0 was preincubated in a 1-ml quartz cuvette (1-cm light path) at 40 °C for 5 min. A 7 µl volume of 200 mM BV was added as the substrate to the cuvette to give a 2 mM final concentration. A 5 µl volume of 10 mM sodium dithionite was added followed by 10 µl 10-fold diluted cell extract. The reaction was started by addition of 70 µl of 20 mM sodium formate. The activity was calculated using the extinction coefficient of BV at 578 nm (8.6 mM−1 cm−1). One unit of enzyme activity is defined as the reduction of 2 µmol of BV per minute. As a positive control, the cell extract of M. maripaludis Mm1328 was used40, which contains FdhAB-type formate dehydrogenase17.
Additional notes on the enzyme activity data shown in Fig. 2
The data in Fig. 2a are consistent with published data and the proteomic data in Fig. 1. The data in Fig. 2b,c are in agreement with the growth rate and the proteomic data shown in Fig. 1. On the basis of these findings, we predicted the presence of the Elp–Hdr–Fmd complex, and this hypothesis was supported by the Hmd+Mtd-dependent Hdr reaction with F420H2 as shown in Fig. 2e. This was also supported by the purification and the cryo-EM analysis of the enzyme complex.
Proteomic analysis
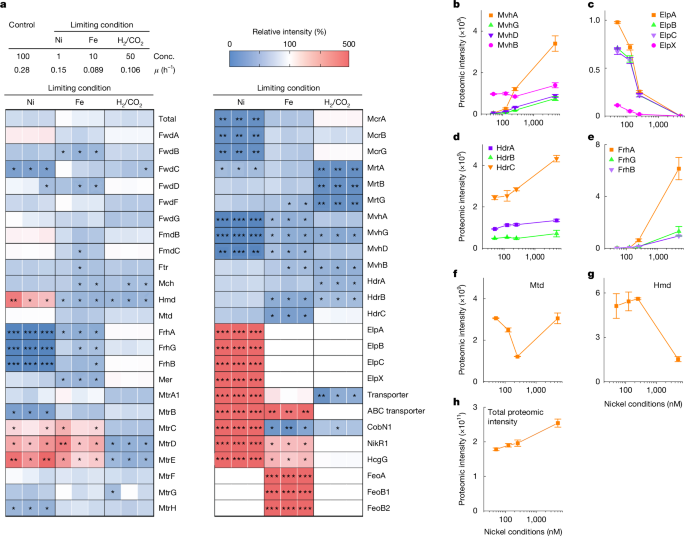
In the proteomic analysis of M. marburgensis, the three cell samples were obtained at three different times from the stable continuous culture. In the case of proteomic analysis of M. thermolithotrophicus, the proteomic samples were obtained from three independent batch cultures under controlled nickel concentrations.
The cell pellets were lysed with 2% sodium N-lauroylsarcosinate at 90 °C and additionally sonicated. The protein concentration was subsequently measured using the bicinchoninic acid method. Carbamidomethylation of the cysteines was performed using 5 mM tris(2-carboxyethyl)phosphine/100 mM ammonium bicarbonate at 90 °C for 10 min and 10 mM iodoacetamide at 25 °C for 30 min. Then 50-µg aliquots of the samples were diluted to 0.5% sodium N-lauroylsarcosinate and digested overnight at 30 °C with trypsin, mass spectrometry (MS)-approved (Serva). Before liquid chromatography–MS analysis, samples were desalted using a Chromabond Spin C18 WP cartridge (Macherey-Nagel) according to the manufacturer’s instructions. Dried and reconstituted peptides were then analysed using liquid chromatography–MS carried out on an Orbitrap Exploris 480 instrument connected to an Ultimate 3000 RSLC nano and a nanospray ion source (Thermo Scientific). Peptide separation was performed on a reverse-phase high-performance liquid chromatography column (75 μm × 42 cm) packed with C18 resin (2.4 μm; Dr. Maisch) run with a 60-min gradient (0.15% formic acid/2% acetonitrile to 0.15% formic acid/50% acetonitrile). MS data were searched against an in-house M. marburgensis protein database using SEQUEST HT embedded into Proteome Discoverer 1.4 software (Thermo Scientific). In the case of the analysis of the protein fraction of the size-exclusion chromatography, the purified fraction was directly used for the MS-based analysis. Proteomic data were quantified using DIA-NN 1.8 software41. To compare the intensity between different proteins, we calculated intensity-based absolute quantification values42.
Protein purification
The frozen M. marburgensis cells (3.5 g) were suspended in 10.5 ml 50 mM Tris/HCl pH 7.6 containing 2 mM dithiothreitol and disrupted as described above. After centrifugation in a Sorvall WX Ultra centrifuge with a T-880 rotor at 41,000 r.p.m. for 30 min at 4 °C, the supernatant containing 150 mg protein, was loaded on a HiTrap Q-HP (5 ml) column, which was equilibrated with 50 mM Tris/HCl pH 7.6 containing 2 mM dithiothreitol (buffer A). The proteins bound on the column were eluted with a step gradient of 50 mM Tris/HCl pH 7.6 containing 2 mM dithiothreitol and 1 M NaCl (buffer B). The step gradient was 30%, 40%, 44%, 48%, 52%, 56%, 60% and 100% buffer B at a 2 ml min−1 flow rate. The 48% or 52% buffer B fraction of the Q-Sepharose chromatography containing most of the Hdr activity was collected and diluted with the same volume of 50 mM Tris/HCl pH 7.6 containing 2 mM dithiothreitol and 1.2 M ammonium sulfate (buffer C). The diluted sample was loaded on a HiTrap Phe-HP (5 ml) column equilibrated with buffer C. The proteins bound on the column were eluted with a step gradient of 50%, 58%, 67%, 83% and 100% buffer A at a 2 ml min−1 flow rate. The elution conditions of Q-Sepharose and Phe-Sepharose columns are according to the previous method used for purification of the Mvh–Hdr complex from M. marburgensis26. The 67% buffer A fraction was exchanged into buffer containing 2 mM dithiothreitol and 150 mM NaCl by an Amicon Ultra-0.5 (3-kDa cutoff) filter. The sample was finally concentrated to about 0.5 ml and applied to a Superose 6 Increase (10/300 GL) size-exclusion column and eluted at a flow rate of 0.5 ml min−1. The eluate was collected in 0.5-ml fractions. The size-exclusion column was calibrated with thyroglobulin (bovine) 670 kDa, γ-globulin (bovine) 158 kDa, ovalbumin (chicken) 44 kDa, myoglobin (horse) 17 kDa, and vitamin B12 1.35 kDa. The standard materials were from Bio-Rad.
Typically, the cell extract containing 150 mg protein with 150 U of BV Hdr activity was fractionated on a HiTrap Q-HP column as described above. The proteins with BV Hdr activity eluted mainly in the 0.52-M NaCl step gradient from the HiTrap Q-HP column. The total yield of BV Hdr activities in the HiTrap Q-HP fractions was 98 U (65% of the loaded sample). The main Hdr fraction at 0.52-M NaCl containing 5.2 mg of protein with 43 U of BV Hdr activity was further fractionated with a HiTrap Phe-HP column, in which the BV Hdr activity was eluted in step gradients containing 0.5-M, 0.4-M and 0.2-M ammonium sulfate as reported previously for the purification of the Mvh–Hdr–Fmd complex from M. marburgensis19. The total yield of BV Hdr activity in the three phenyl-Sepharose fractions was 36 U (84% of the loaded sample). We used the 0.4-M ammonium sulfate fraction containing 0.9 mg protein with 15 U BV-Hdr activity for further fractionation on a Superose 6 Increase column. The elution profile of the BV Hdr and F420 Hdr activity and the profile of the F420-dependent Hdr activity are shown in Fig. 2f, and SDS–polyacrylamide gel electrophoresis (PAGE) analysis is shown in Extended Data Fig. 2 and Supplementary Fig. 7. The fractions with BV Hdr activity from the Superose 6 Increase contained 0.68 mg protein and 11 U BV Hdr activity (73% of the loaded sample). The Elp–Hdr–Fmd complexes were purified seven times. We performed SDS–PAGE analysis four times and proteomic analysis once (Extended Data Table 1 and Extended Data Fig. 3a–d). The SDS–PAGE data supported the reproducibility of the purification and the proteomic analysis.
Cryo-EM sample preparation and data collection
The cryo-EM sample preparation was performed immediately after the Superose 6 Increase (10/300 GL) purification step inside an anaerobic chamber (<1 ppm O2; Coy Laboratory Products). The fraction corresponding to the 1-MDa peak was used for freezing. For each grid (glow-discharged UltrAuFoil 1.2/1.3 300 mesh), 3 µl of the sample (1 mg ml−1) was applied, blotted for 4 s with Whatman 595 filter paper (Sigma-Aldrich) at 4 °C under 100% humidity, and plunge-frozen in liquid ethane using a Vitrobot Mark IV (Thermo Fisher Scientific). The dataset was corrected using aberration-free image shift at a Titan Krios G3i equipped with a BioQuantum energy filter and a K3 detector (Gatan) at an image pixel size of 0.837 Å per pixel. Dose-fractionated videos were collected, with a total dose of 65 electrons per square ångström spread over 65 fractions and a defocus range between −0.8 µm and −2.4 µm. EPU v3.6 (Thermo Fisher Scientific) was used for automated data acquisition of 7,768 videos.
Image processing and model building
A detailed workflow of the steps for data processing is shown in Extended Data Fig. 4. An initial fast data screening was performed on-the-fly using CryoSPARC Live43. Videos were motion-corrected and defocus parameters were calculated using the Patch Motion correction and CTF estimation tools, respectively. Corrected micrographs were then selected on the basis of a maximum 4.0 Å CTF resolution estimate, and micrographs with substantial crystalline ice contamination were manually removed. A total of 6,488 curated micrographs were then exported to CryoSPARC v3.1 (ref. 43) for data processing. Blob picker, template picker and a trained Topaz model44 were used one after another to reach optimal particle picking. An initial set of particles was obtained using the Blob picker (minimum and maximum diameters of 60 Å and 550 Å, 500 local maxima considered), and the extracted particles (2.7 million particles, 500-pixel box downsampled to 126 pixels) were subjected to two rounds of 2D classification. A total of 31 class averages from 351,000 particles were selected and used as templates for the template picker. The initially picked particles (551,000) were extracted (500-pixel box, downsampled to 150 pixels) and subjected to two rounds of 2D classification to remove bad particles. A total of 350,000 particles corresponding to 110 2D classes were then randomized, and a subset of 20,000 was used to train a Topaz model (downsampling factor 8, 500 expected particles per micrograph, ResNet8). The Topaz Extract tool (radius 15, 200 iterations, downsampling 8) was used to pick 1.3 million particles that were extracted (416-pixel box, no downsampling) and used for 2D classification. A total of 1.1 million particles corresponding to 108 classes were selected for further processing. Three ab initio models were obtained using the ab initio reconstruction job and used as templates for heterogeneous refinement (C1, no downsampling). The map of one of the three classes showed a clear density for Hdr(ABC)2 and blurred regions for the flexible Elp arms. No density could be observed for Fmd.
To continue with the processing using RELION 4 (ref. 45), particle coordinates of 493,925 particles belonging to the good class from heterogeneous refinement were exported to RELION format using pyem46. Before particle extraction, the raw videos were motion-corrected and dose-weighted with RELION’s MotionCor2 implementation47 using 5 × 5 patches, and CTF resolution was estimated using CTFFind4.1 (ref. 48). The particles were extracted in a box of 416 pixels, downsampled to 384 pixels and reimported into CryoSPARC. A masked 3D refinement was performed with C2 symmetry, giving a map at 2.48 Å resolution. CTF parameters were refined using local and global CTF refinement tools, and a map of Hdr(ABC)2 (without the N- and C-terminal flexible HdrA domains forming part of the flexible Elp arms) could be obtained at 2.04 Å after homogeneous refinement with C2 symmetry applied. Data processing was then performed separately for the Hdr(ABC)2 region and the flexible Elp arms.
For the Elp arms, the C2-refined particles (493,925) were converted to the Relion format using Pyem and then symmetry-expanded using relion_particle_symmetry_expand. A model of the Fdh–Hdr–Fmd complex from M. hungatei (PDB accession code 7BKC)18 was aligned to the Hdr(ABC)2 map, Fmd subunits were deleted, and a 30-Å low-pass-filtered volume was generated using the molmap tool of ChimeraX49. The region corresponding to the FdhAB–MvhD mobile arm was used to create a 30 Å low-pass-filtered mask. The reference volume and mask were used for a focused 3D classification of the symmetry-expanded particles without alignment (T = 4, 3 classes, 25 iterations). The three classes obtained corresponded to the Hdr region without any apparent density for the mobile arm (676,764 particles), and two clearly different states of the Elp arm (state 1, 76,528 particles; state 2, 234,858 particles). These conformational states are very similar to conformational states 1 and 2 of the M. hungatei Fdh–Hdr–Fmd complex18. Particles corresponding to each state of the Elp arms were reimported into CryoSPARC for masked local and CTF refinements using masks obtained from the 3D classification output volumes. Then, the particles were reimported into Relion for Bayesian polishing and re-extracted with a 448-pixel box size without downsampling. The particles were reimported into CryoSPARC for final local and CTF refinements. For each conformation, three different maps were obtained: one consensus map obtained using a mask containing the arm and the Hdr region, and two focused maps obtained using masks for the arm (mobile-arm-focused maps), and for the Hdr(ABC)2 dimer separately (Hdr-focused maps). For state 1, a map of Elp at 2.4 Å, a map of Hdr at 2.1 Å and a consensus map at 2.47 Å were obtained. For state 2, a map of the arm at 2.2 Å, a map of Hdr at 2.3 Å and a consensus map at 2.3 Å were obtained (Extended Data Fig. 4 and Supplementary Figs. 3 and 4).
For the analysis of MvhB, we used Relion 5.0 (ref. 50) to perform focused 3D classification (Blush regularization, T = 4, 3 classes, 25 iterations)51, of the symmetry-expanded particles without alignment. We used the same reference map as for the mobile arms (see above), and we created a mask (30 Å low-pass filter, 4-pixel extension and 12-pixel soft-padding) from the entire MvhB subunit of the AF3 HdrA–MvhB–FmdF complex (Extended Data Fig. 7) after alignment with the reference map. One of the three classes, corresponding to around 12% of the particles (119,398), showed an extra density that could not be fitted to the inserted ferredoxin-like domain of HdrA. The particles were further refined using 3D refinement with 1.8 Å local and angular searches and Blush regularization. A 3 Å-resolution map could be obtained. The particles were then imported into CryoSPARC v4.5.1 and further subjected to local refinement using a wider mask including HdrA. Then, the particles were subjected to local and global CTF refinements, and a final local refinement was performed (Extended Data Fig. 7c), which resulted in a 2.54 Å map showing an additional density attached to the inserted ferredoxin-like domain of HdrA.
For Hdr(ABC)2, the C2-refined particles (493,925) were reimported into Relion for Bayesian polishing (448-pixel box size, no downsampling). The particles were reimported into CryoSPARC and used for several rounds of focused local and CTF refinements until a focused map at 1.85 Å resolution was obtained.
The focused maps corresponding to the Hdr(ABC)2 map at 1.85 Å and the Elp arm in conformational state 2 at 2.2 Å were used for automatic model building using the machine-learning-based tool ModelAngelo52. The program COOT53 was then used to place cofactors and to inspect and manually adjust the models. Then, for each conformational state, the models of each region were rigid-body-fitted into the consensus map to generate combined models of Hdr(ABC)2 plus one Elp arm (in state 1 or under state 2) in ChimeraX. Finally, composite maps of the subcomplex comprising Hdr(ABC)2 plus one Elp arm were generated with the tool phenix.combine_focused_maps54 using as inputs: the consensus maps, the focused maps and the combined models. Furthermore, a combined map of the Elp–Hdr dimer was generated using the combined map of Elp–Hdr in state 2 and a dimer model (ElpABC–HdrABC)2 in conformational state 2, using the tool phenix.combine_focused_maps. Iterative rounds of PHENIX real-space refinement55 and manual inspection and readjustment in COOT were performed to optimize the model stereochemistry and the fit to the cryo-EM density map as assessed with PHENIX, MolProbity56 and Q-score57. Root mean square deviation values were calculated by the mmaker command of ChimeraX49.
AF structural modelling
The HdrA–MvhB–FmdF complex of M. marburgensis (Extended Data Fig. 7) was predicted with the AlphaFold3.0 server33. The sequences used for the prediction were obtained from the UniProtKB database: M. marburgensis HdrA (Q50756), MvhB (P60232) and FmdF (D9PU52). Output models were assessed to determine whether a credible complex was generated, and subunit interfaces were inspected manually for surface complementarity and the absence of clashing atoms. When needed, cofactors were added to the predicted models. For HdrA and FmdF subunits, the AF3 models were aligned to experimental models of HdrA (this paper, PDB accession code 8RWN) and FmdF (Methanothermobacter wolfeii, PDB accession code 5T61), and cofactors were added to the corresponding positions. For MvhB, [4Fe–4S] clusters were fitted at the predicted [4Fe–4S]-binding sites, which could be identified by the location of the coordinating cysteines.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.