Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925â1936 (2016).
Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 29, 1541â1547 (2018).
Johnston, S. et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 5 (2019).
Tripathy, D. et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 19, 904â915 (2018).
Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 17, 425â439 (2016).
Slamon, D. J. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 36, 2465â2472 (2018).
Sledge, G. W. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2â advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875â2884 (2017).
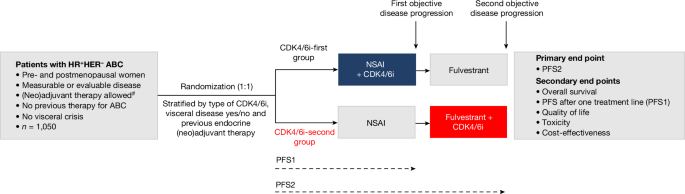
van Ommen-Nijhof, A. et al. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancerâthe SONIA study: study protocol for a randomized controlled trial. BMC Cancer 18, 1146 (2018).
Sledge, G. W. et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 6, 116â124 (2020).
Hortobagyi, G. N. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 386, 942â950 (2022).
Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 382, 514â524 (2020).
Lu, Y. S. et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2â advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin. Cancer Res. 28, 851â859 (2022).
Gradishar, W. J. et al. NCCN Guidelines Insights: Breast cancer, Version 4.2023. J. Natl Compr. Cancer Netw.21, 594â608 (2023).
Kümler, I., Knoop, A. S., Jessing, C. A., Ejlertsen, B. & Nielsen, D. L. Review of hormone-based treatments in postmenopausal patients with advanced breast cancer focusing on aromatase inhibitors and fulvestrant. ESMO Open 1, e000062 (2016).
Yang, C. et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36, 2255â2264 (2017).
Park, Y. H. et al. Longitudinal multi-omics study of palbociclib resistance in HR-positive/HER2-negative metastatic breast cancer. Genome Med. 15, 55 (2023).
Gyawali, B. et al. Problematic crossovers in cancer drug trials. Nat. Rev. Clin. Oncol. 20, 815â816 (2023).
Spring, L. M. et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 395, 817â827 (2020). A.
Cherny, N. I. et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 26, 1547â1573 (2015).
G-standaard, Tarieven Januari (Z-index, 2023).
G-standaard, Tarieven Januari (Z-index, 2019).
Centers for Medicare & Medicaid Services. Medicare Part D Spending by Drug (Centers for Medicare & Medicaid Services, accessed 8 May 2024); data.cms.gov/summary-statistics-on-use-and-payments/medicare-medicaid-spending-by-drug/medicaid-spending-by-drug.
Johnston, S. et al. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. NPJ Breast Cancer 7, 80 (2021).
Rugo, H. S. et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 174, 719â729 (2019).
Di Lauro, V. et al. Health-related quality of life in breast cancer patients treated with CDK4/6 inhibitors: a systematic review. ESMO Open 7, 100629 (2022).
Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 379, 1926â1936 (2018).
Finn, S. R. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptorâpositive/human epidermal growth factor receptor 2ânegative advanced breast cancer (ER+/HER2âABC): Analyses from PALOMA-2. J. Clin. Oncol. 40, LBA1003 (2022).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929â1940 (2019).
Turner, N. C. et al. Capivasertib in hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 388, 2058â2070 (2023).
Bidard, F. C. et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J. Clin. Oncol. 40, 3246â3256 (2022).
Garcia-Fructuoso, I., Gomez-Bravo, R. & Schettini, F. Integrating new oral selective oestrogen receptor degraders in the breast cancer treatment. Curr. Opin. Oncol. 34, 635â642 (2022).
Woodford, R. et al. Validity and efficiency of progression-free survival-2 as a surrogate end point for overall survival in advanced cancer randomized trials. JCO Precis. Oncol. 8, e2300296 (2024).
European Medicines Agency. Appendix 1 to the Guideline on the Evaluation of Anticancer Medicinal Products in Man (EMA, 2012).
Fojo, T. & Simon, R. M. Inappropriate censoring in Kaplan-Meier analyses. Lancet Oncol. 22, 1358â1360 (2021).
Gyawali, B. et al. Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring. ESMO Open 6, 100117 (2021).
Committee for Medicinal Products for Human Use. Guideline on the Choice of the Non-Inferiority Margin (EMA, 2005).
Tannock, I. F. et al. The tyranny of non-inferiority trials. Lancet Oncol. 25, e520âe525 (2024).
Committee for Medicinal Products for Human Use. Concept Paper for the Development of a Guideline on Non-Inferiority and Equivalence Comparisons in Clinical Trials (EMA, 2024).
André, F. et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann. Oncol. 32, 208â217 (2021).
Robson, M. E. et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physicianâs choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 30, 558â566 (2019).
Casparie, M. et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 29, 19â24 (2007).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228â247 (2009).
Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 (National Cancer Institute, 2010).
Brady, M. J. et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J. Clin. Oncol. 15, 974â986 (1997).
Eton, D. T. et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J. Clin. Epidemiol. 57, 898â910 (2004).