Animals
Experiments were carried out in male and female mice (C57BL/6J background, wild type, Rbp4-Cre (MMRRC, 031125-UCD) and vGAT-Cre (JAX, 028862)) maintained on a mixed genetic background (129/C57BL6), aged 2–6 months at the start of the experiments. The mice were maintained at 22 ± 1 °C) at relative humidity ranging from 46–65% and under a 12 h–12 h light–dark cycle. Transgenic experimental animals were heterozygous from a backcross to C57BL6. They originated from different litters, were randomly allocated to experimental groups and were identified by earmarks. All of the procedures pertaining to housing, surgery, behavioural experiments and euthanasia were approved by the Cantonal Veterinary Office Basel-Stadt and performed in compliance with the Swiss Veterinary Law guidelines.
Viral tools
The following adeno-associated viruses (AAVs) and rabies viruses were used for anatomical and functional experiments: AAV-flex-SynGFP (referred to as AAV-flex-SynTag)53, CVS-N2c-nl.mCherry-FlpO (referred to as rabies-nTag)54 (Addgene, 172378), SiR-N2c-iCre (referred to as rabies-Cre)55, AAV-EF1a-double floxed-hChR2(H134R)-EYFP (Addgene, 20298), AAV-Ef1a-DIO-ChRmine-mScarlet (Addgene, 130998), AAV-iCre-H2B-GFP, AAV-FRT-ChRmine-p2a-oScarlet, AAV-flex-FlpO-H2B-V5 (all generated as described previously)26. To infect neurons through local infection, a 2.9 serotype plasmid was used for production. For retrograde targeting of neurons by means of axonal infection, either rabies virus for anatomical experiments or AAV2.1156 for functional experiments was used. All AAVs used in this study were produced according to standard protocols. Genomic titres for AAVs were between 1 × 1012 and 1 × 1014 genome copies per ml, while for rabies between 1 × 107 and 1 × 108 genome copies per ml.
Surgical procedures
Buprenorphine (Temgesic, 0.1 mg per kg) was applied subcutaneously as pre-emptive analgesia half an hour before the beginning of surgery. Mice were anaesthetized with 2–3% isoflurane using oxygen as a gas carrier. Once deeply anaesthetized, the mice were transferred to the stereotaxic frame under 1–2% isoflurane. Anaesthesia was kept constant by regulating the isoflurane concentration. Surgical equipment was disinfected, and a heating pad was used during the surgical procedures to avoid body temperature dropping. Eyes were protected from dehydration with ocular gel. Mice were injected with a mixture (50:50) of lidocaine (10 mg per kg) and ropivacaine (Naropin, 3 mg per kg) in the area of the surgery to reduce post-operative pain. Once anaesthetized, the skin was shaved and disinfected. After surgery, buprenorphine (0.1 mg per kg) was applied subcutaneously on the day of the surgery, followed by subcutaneous injection of meloxicam (5 mg per kg) at awakening to ensure analgesia and for the next 2 days at an interval of 24 h or administration of carprofen (10 mg per kg) in the drinking water from the day preceding surgery to 2 days after. Application of viruses, implantation of electrophysiological recording probes and implantation of optic fibres were directed to the target brain regions using high-precision stereotaxic instruments (Kopf Instruments, Model 1900). Stereotaxic coordinates for brain injections are defined as anteroposterior (AP), mediolateral (ML) and dorsoventral (DV) (approximate injection volumes: 20–100 nl), taking bregma or lambda as a reference for the AP and ML axes (SNr: −3.6 mm AP from bregma, 1.6 mm ML, 4–4.5 mm DV from dura mater; latRM: −1.95 mm AP from lambda, 1.5 mm ML, 4.5 mm DV from dura mater; PPN: −0.5 mm AP from lambda, 1.1 mm ML, 3 mm DV from dura mater).
Immunohistochemistry and microscopy
After termination of in vivo experiments, mice were euthanized, and brains and spinal cords were collected for histological processing. In brief, animals were anaesthetized with a ketamine–xylazine solution and transcardially perfused with PBS, followed by a solution containing 4% paraformaldehyde in PBS. The brain and spinal cord were dissected, post-fixed overnight in 4% paraformaldehyde and incubated in 30% sucrose (w/v) in PBS for at least 2 days before cryopreservation. Coronal brain tissue sections were cut on a Cryostat at a thickness of 80 μm. Floating sections were collected in sequential order into individual wells and incubated for 1 h in blocking solution (1% BSA, 0.2% Triton X-100, PBS). Primary antibodies were then applied in blocking solution and incubated for 1–3 days at 4 °C. Fluorophore-coupled secondary antibodies (Jackson or Invitrogen) were applied to floating sections after extensive washing and incubated for 1 day at 4 °C. The sections were then washed and mounted with anti-bleach preservative medium on slides in sequential rostrocaudal order. Primary antibodies and respective dilutions used in this study were as follows: chicken anti-GFP (1:2,000, Invitrogen, A10262), rabbit anti-RFP (1:5,000, Rockland, 600-401-379), chicken anti-TH (1:500, Neuromics, CH22122), goat anti-ChAT (1:500, Millipore, AB144P). The following secondary antibodies were used all diluted 1:1,000: donkey anti-rabbit Cy3 (Jackson Immuno Research, 711-165-152), donkey anti-goat Cy5 (Invitrogen, A-21447), donkey anti-chicken 488 (Jackson Immuno Research, 703-545-155), donkey anti-chicken Cy5 (Jackson Immuno Research, 703-605-155), donkey anti-goat 488 (Invitrogen, A-11055). For low-resolution overview imaging, slides were scanned with an Axioscan light microscope (Zeiss). For higher-resolution imaging used for anterograde tracing (Extended Data Fig. 7b), we used the Axio Imager M2 microscope (Zeiss) with a Yokogawa CSU W1 dual-camera T2 spinning-disk confocal scanning unit.
Behavioural experiments
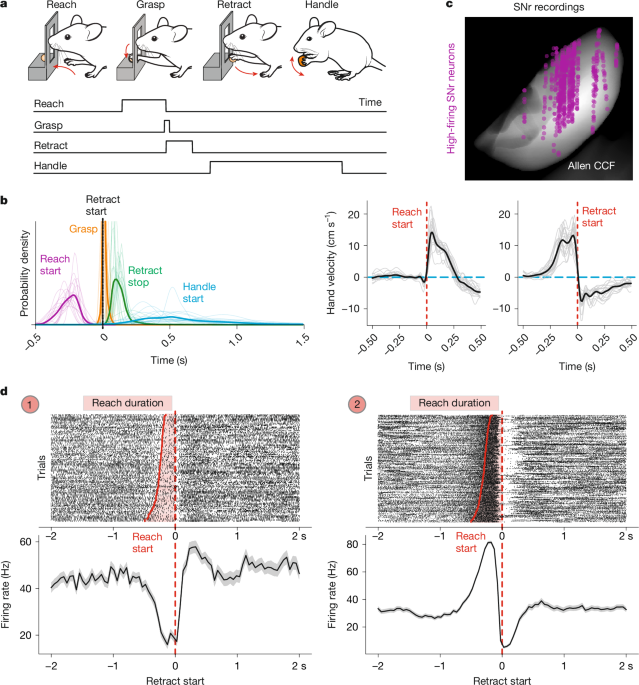
For the pellet reaching and handling task, food-restricted mice were placed into a custom-made chamber containing a slit and trained to protrude the arm through the slit, reaching for a food reward. The body weight of mice and food consumption was monitored daily to not drop below 80% of the baseline weight. Mice were encouraged to use the forelimb for reaching trials by placing food pellets at a consistent position outside the slit and were trained for 5–10 days. All mice recorded produced stereotyped forelimb movement kinematics after training (Fig. 1b).
For the lever-pressing task, water-restricted mice were placed into a custom-made chamber containing a slit and were trained to protrude their arm through the slit, to press a custom-made lever delivering a digital signal to synchronize with the rest of the equipment. The task was programmed using the visual reactive programming language Bonsai57. The visual signal was delivered using a small blue LED. Water rewards were delivered through a blunt feeding needle (FST, 18061-10) and a solenoid valve (The Lee Company, LHDA0531115H). Mice were trained for 2–4 weeks in total, with a 1-day break minimally every 14 days. After training, all of the mice responded to the visual signal with a lever press in the response window as seen in the distribution of the press latency and ethograms (Fig. 4c,e and Extended Data Fig. 5b). The body weight of mice and water consumption was monitored daily to not drop below 80% of the baseline weight. Videos were recorded from below and side for pose estimation with Basler cameras (Ace 2 series with Pylon software and interfacing with Arduino IDE) for the lever-pressing and pellet-retrieval tasks.
Electrophysiological recordings
To perform in vivo extracellular electrophysiological recordings, Neuropixels probes (IMEC, NP1.0 or 2.0)58,59 were implanted in the midbrain to target the SNr. Before probe implantation, Neuropixels probes were mounted onto 3D printed fixtures (ATLAS Neuroengineering)60. We confirmed correct probe placement and location of recording sites after termination of experiments, using immunohistochemistry and probe coating with a thin layer of DiI (Invitrogen) and all probes implanted had units putatively isolated in the SNr (Fig. 1c). For Neuropixels probes, we used the SpikeGLX software to record electrophysiological data synchronized with camera timestamps. When applicable, task-related signals (cue, lever, reward valve) and laser pulse signals were collected using the National Instruments PXIe-6341 multifunction IO module through the BNC-2110 breakout box with the National Instruments PXIe/PCIe-8281 controller module.
Optogenetic perturbation experiments
Optogenetic stimulation was performed using a PlexBright Radiant Optogenetic Stimulation System (Plexon) in combination with lasers (Cobolt 06-MLD, 473 nm; Cobolt 06-DPL, 532 nm). Light was delivered through a patch cord and rotary joint (Doric Lenses) connected to the animal’s optic fibre (Doric Lenses C60 or FLT). The laser intensity was measured at the beginning of every session using an optical power meter (Thorlabs) on the tip of an optic fibre with the same characteristics as the one implanted to ensure consistent stimulation power. Optogenetic stimulation was provided at the cue offset (100 ms from presentation) on 40–50% of randomly selected trials. A continuous light pulse was used when stimulating the PPN>SNr neurons as described previously41. In the case of the optogenetic activation of latRM projecting SNr neurons, a 100 Hz 50% duty cycle signal was used to drive the laser to activate the SNr neurons. Fibre tip powers used in these experiments ranged between 1 mW and 20 mW. For the stimulation of the PPN>SNr neurons, we injected AAV2.9-Ef1a-DIO-ChRmine-mScarlet or AAV2.9-EF1a-double-floxed-hChR2(H134R)-eYFP in the PPN of Rbp4-cre mice. In the case of the stimulation of the SNr>latRM neurons, we injected AAV2.11-iCre-H2B-GFP into the latRM and AAV2.9-Ef1a-DIO-ChRmine-mScarlet in the SNr of WT mice or AAV2.11-flex-FlpO-H2B-V5 in the latRM and AAV2.9-FRT-ChRmine-p2a-oScarlet in the SNr of Vgat-cre mice. This dual strategy was motivated by previous findings reporting paradoxical silencing of SNr neurons after optogenetic activation due to local inhibitory collaterals61. To control for the effects of SNr>latRM neuron stimulation, we performed the same perturbation as in the lever-pressing task in an open-field arena in a closed-loop with locomotion using Bonsai57. Specifically, light was delivered when the mouse crossed a locomotor speed (centroid speed) threshold of 10 cm s−1 for 150 ms. The results of these experiments are shown in Extended Data Fig. 9, revealing no significant effect on locomotor speed (for comparison see fig. 6f in ref. 41).
Quantification and data analysis
Anatomical reconstructions and data analysis
Maximum-intensity projections of coronal brain sections obtained from a confocal microscope using a ×20 objective (anterograde tracing) or from a light microscope with a ×5 objective (nucleus segmentation) were manually linearly registered and aligned to the Allen CCF using an adapted version of AP_histology (https://github.com/petersaj/AP_histology).
Nuclei in fluorescence images were detected using a custom implementation of stardist in ImageJ (https://imagej.net/plugins/stardist) and their centroid coordinates were transformed into CCF coordinates for visualization over contour plots of the CCF annotation volume. For each CCF level, nuclei from the preceding and following 50 μm AP were gathered and plotted and their 2D density estimated at each pixel on a CCF level using kernel density estimation (Extended Data Fig. 1b). Projection density was quantified starting from a thresholding step where the SynTag signal was binarized from the background and manually curated to remove autofluorescent artifacts from immunohistochemical processing. The density of SynTag-positive pixels falling in each CCF voxel (resolution of 80 × 10 × 10 μm, AP × DV × ML) was then computed thereby generating a 3D volume in CCF space of SynTag density. The obtained density was filtered using a Gaussian kernel with a s.d. of 2,3,3 (AP × DV × ML) and truncated at 2 s.d. on each dimension. The obtained density array was then zoomed using second-order spline interpolation to reach a final voxel size of 10 × 10 × 10 μm (AP × DV × ML) and normalized to between 0 and 1. Projection density was averaged across mice, scaled and then displayed over equally spaced CCF coronal levels, plotting the respective CCF annotation volume as a contour plot (Extended Data Fig. 7).
Analysis of Allen Brain Institute BrainMap data
To quantify brainstem projections of SNr neurons, we used experiments numbers 100141993, 175263063 and 299895444 from the Allen Brain Connectivity atlas (https://connectivity.brain-map.org/)62. Volumetric projection density data were downloaded as nrrd files using the Allen SDK (https://allensdk.readthedocs.io). Projection density was averaged across experiments, scaled and then visualized in equally spaced CCF coronal levels plotting the respective annotation volume as a contour plot.
Behavioural analysis in the forelimb reaching task
For analysis of forelimb movements executed during the forelimb task, we applied deep-neural-network-based markerless pose estimation using DeepLabCut63 coupled with high-speed videography of the bottom view of the mouse at 100 fps to track the moving hand and the slit. We used a DeepLabCut model trained on frames of different videos of mice from the bottom view over many similar behavioural experiments35. Obtained predictions were median-filtered with a filter size of 5 or filtered using sosfiltfilt in Python with an order of 4 and frequency of 15. Reaches were detected as peaks in the position of the hand over the slit with a prominence threshold (slit crossings) based on the two-dimensional position of the hand and slit as seen from the bottom view. The retraction start was defined as the moment of maximum extension (also corresponding to reach stop), and the reach start was detected by rolling back from the retraction start in a time window of 0.5 s to the moment when the hand velocity along the main reach direction decreased below a threshold of 1 pixel per second. The retraction stop was detected rolling forward from the retraction start as the moment when the hand velocity along the main reach direction increased above a threshold of −1 pixel per second. We identified isolated reaches as those separated from previous and next reach by a minimum of 0.75 s. Average velocity profiles were computed for each mouse and then averaged across mice for Fig. 1b. Handle start, manipulation start and stop were manually detected as the hand to mouth movement that mice perform to start consuming the pellet, the downwards movement away from the mouth during handling that precedes pellet manipulation and regripping, and an upward movement towards the mouth that precedes chewing, respectively38. To compute modulation during task time windows, we isolated select time windows, overall capturing the sequence of produced movements in the task (Supplementary Video 1): (1) reach start, from −150ms to +25 ms from the detected reach start64; (2) reach, from reach start to retraction start; (3) reach distal, from slit crossing to retraction start, to capture the lateral forelimb movement towards the pellet; (4) retraction start, a 50 ms window starting at the retraction start encompassing finger closure; (5) retraction, from retraction start to retraction stop; (6) handle start, the 100 ms window centred at handle start; (7) manipulation start, the 50 ms after the annotated manipulation start timepoint; (8) manipulation, from start to stop; and (9) manipulation stop, the 50 ms after the annotated manipulation stop timepoint. The repeated reach trials were identified as those pairs of detected reaches that occurred within 0.3–0.6 s of each other, comparing the detected retraction start timestamps (Fig. 2d and Extended Data Fig. 2d). To stratify trials in which different movement phases were selectively altered we used either kinematics tracking or manual annotation. To identify trials with different reach duration, we identified in each animal the trials that lay between the 1st and 25th percentile of reach durations (short reaches) and 73rd to 97th percentile (long reaches) (Supplementary Video 2). We did not use the extremes of the data to avoid capturing idiosyncratic trials. Abbreviated reaches were defined as those trials in which the arm extended maximally 0.45 cm past the slit on the major movement axis and 0.25 cm on the minor axis and thus did not reach the pellet (Supplementary Video 2). Reach trials followed by pellet retrieval were identified manually. These trials, in which the reach was followed by handling, were contrasted to the remaining detected isolated reaches in an analysis comparing the activity between these trial types.
Electrophysiological data analysis
All data were processed using the ecephys spike sorting modules for SpikeGLX (https://github.com/jenniferColonell/ecephys_spike_sorting). In brief, data collected from SpikeGLX were first processed using the CatGT module to apply demultiplexing corrections, removing electrical artefacts (using ‘gfix’) and high-pass filtering the data. Moreover, the edges of the synchronization pulses from the IMEC base station on which the Neuropixels data were recorded, the camera exposure pulses and laser pulses were extracted. Subsequently, using the Kilosort helper module, channels with a firing rate below 0.05 Hz were excluded as noisy channels and the channel map for the spatial location of the remaining channels was constructed using the metadata from the recordings. We used Bank 0 on the Neuropixels 1.0 to record from the ventral-most 384 channels on the probe. Subsequently, Kilosort3 was run on the data. After the sorting, TPrime module helped to synchronize all of the datastreams precisely, with the IMEC base station recording as the reference time stream, using the synchronization pulse recorded on both the multifunctional IO device and the IMEC base station. To align a probe tract to anatomy, we registered the probe tract identified by DiI signal to mark the Neuropixels probes, to the Allen CCF (https://github.com/petersaj/AP_histology). Using the ephys alignment tool from the International Brain Laboratory (https://github.com/int-brain-lab/iblapps/wiki), we aligned the electrophysiological features from the data to the anatomical landmarks to obtain the precise probe trajectory and channel locations in CCF coordinates. We then performed manual curation of the output of Kilosort3 in Phy 2 to obtain isolated single units. Single units were identified in anatomical CCF space based on their peak channel and plotted over the closest 100 μm spaced CCF level. For SNr recordings, based on extensive previous literature concerning the tonic activity of neurons9,10,11,12,13,14,15,16,17,18,19,20,29,31,36,45,65,66,67,68,69, we isolated neurons with a mean spike rate over the entire recording session higher than 5 Hz. This resulted in a dataset of 646 units across 17 mice for the pellet-reaching task and 184 neurons across 5 mice for the lever-pressing task with optogenetic perturbation. For the remaining midbrain and latRM electrophysiology data, all curated single units were included for analysis (2,197 neurons across 17 mice in the remaining midbrain and 709 neurons across 8 mice in the latRM). For the latRM, neuronal recordings from four mice were part of a previously published dataset35, while four mice were recorded for this work. The firing rate was binned into bins of 50 ms for subsequent plotting of single-neuron PETHs. For heat maps and average firing rates across populations of SNr neurons in Figs. 2 and 3 and the related Extended Data Figs. 2 and 3, we computed the firing rate of each neuron in 5 ms bins with a Gaussian filter with a sigma of 10 and a size of 100 ms. Average firing rates in 2 s windows surrounding retraction start were concatenated across all trial types considered in the manuscript (see the ‘Behavioural analysis in the forelimb reaching task’ section) and z-scored for display and further analysis of the effects movement variation on neuronal activity.
Task event modulation
To identify SNr neurons modulating firing rate aligned to different phases of the analysed forelimb tasks, we calculated modulation during the task time windows defined in the ‘Behavioural analysis in the forelimb reaching task’ section above. For each neuron and each time window, we computed the mean spike rate during the window across N trials and defined a null distribution of mean firing rates computed over 1,000 groups of N random windows. We defined modulation as the difference between the mean spike rate during the window and the median of the null distribution of mean firing rates, normalized to the sum of these two variables, to mitigate the effects of differences in baseline spiking. To compute the statistical significance of the computed modulation, we ranked the mean spike rate in the task time window with respect to the null distribution and considered as significant only modulations higher than an absolute 0.025 threshold, with a rank either between the 0th and the 1st percentile or between the 99th and 100th percentile of the null distribution for negative and positive modulation, respectively.
Correlational structure of neuronal activity during repeated reach events
To evaluate the neuron-to-neuron correlational structure of neuronal activity, we correlated the average firing rates of all recorded neurons for each single mouse in a −2s to +2 s window around the relevant timestamp (for example, first retraction start or random timestamps in the session). The resulting pairwise Pearson correlation across neurons was plotted in the form of a heat map in Extended Data Fig. 2e. We then regressed the obtained pairwise neuron correlations across timestamps and obtained the slope and R value of the fit across pairs of timestamps to generate summary bar plots in Extended Data Fig. 2e.
Analysis of changes in spiking activity in movement variation trials
To discern the effects of trial type on the firing rate across all examined pairs of trials (Fig. 3a; see the ‘Behavioural analysis in the forelimb reaching task’ section), we compared firing rates of different neuronal populations to a null distribution of average firing rates computed over 1,000 random groups of trials sampled from each trial type. Given N trials of type 1 and M trials of type 2, with N < M, we generated 1,000 random groups of N trials, equally sampling from trial type 1 and 2. For each neuron, we computed the average firing rate for each of these 1,000 random groups, thereby obtaining a null distribution of firing rates aligned to the retraction start, and z-scored using the mean and s.d. obtained across all trial types considered in the Article (see the ‘Behavioural analysis in the forelimb reaching task’ section). For each neuronal population, we identified z-scored average firing rate windows longer than 50 ms in which the difference in firing rate in the two trial types was larger than the 99.9% confidence interval of the null distribution of average firing rates for that neuronal population. In practice, we identified data timepoints at which the average firing rate of the population in one trial type was below the 0.05th percentile and the other trial type above the 99.95th percentile of the null distribution of average firing rates for that neuronal population.
This analysis was carried out on specific populations of single units: (1) SNr neurons pausing throughout arm extension, that is, reach and reach distal time windows and not negatively modulated during handling-related time windows (handle start, manipulation start, manipulation stop, manipulation); (2) SNr neurons negatively modulated at the retraction start but not during the reach time window; (3) SNr neurons negatively modulated at the handle start but not during any proximal task time window (reach start to retract). For Extended Data Fig. 3i, we isolated a subset of neuronal population 2, the SNr neurons negatively modulated at retraction start and during at least one handling-related time window.
Analysis of neuronal recordings during optogenetic perturbation
To quantify the response of SNr neurons to optogenetic excitation, in each mouse, we computed the average laser-evoked and cue-evoked firing rate of each SNr neuron. Obtained arrays were concatenated together and z-scored for each single neuron using the mean and s.d. of firing rates in control trials. We then averaged across the recorded SNr population for each recorded mouse (Fig. 4c) and, subsequently, across mice (Fig. 4d (top)). We then performed the same operation on the lever-press rate computed in 200 ms bins. In brief, we computed the average laser-evoked and control press rates and plotted their difference as the average across mice. We also computed the fraction of trials with a lever press in the response time in control and stimulation trials for each mouse and plotted the ratio of laser over control response rates as control normalized response rate (Extended Data Fig. 5a). To further quantify behavioural effects of laser exposure we compared the distribution of press latencies in laser versus control trials using Kolmogorov–Smirnov tests on single mice and plotted the single mouse and average cumulative distribution across mice (Fig. 4e) and experiments (Extended Data Fig. 5b). To regress laser response against median increase in latency to press the lever, for each mouse we computed the time during which laser-evoked SNr population response was at least 1 s.d. above the control (s.d. of control responses).
To quantify lever-press-related pauses in laser and control trials (Fig. 4f), we isolated neurons that were negatively modulated to the press in a 200 ms window centred around the lever press in control trials. We calculated the modulation for each trial of the behaviour for each neuron as the average firing rate in the behaviour window in that trial above the baseline. For the modulation to the lever press, we used the 200 ms window preceding the presentation of the cue as the baseline. The distribution of modulation indices (MIs) obtained was compared to the distribution of MIs obtained for the same neuron from a minimum of 200 random timepoints sampled over the entire recording session or as many as those of the behaviour in a time window equal to the one used for the behaviour in question using a Mann–Whitney U-test. A neuron was classified as modulated when it had P < 0.05 and the average of the MI over all of the trials of the behaviour was smaller than 0 for negative modulation.
Connectivity analysis across SNr and midbrain neurons
To identify connected pairs of neurons between SNr and midbrain, we used Python and the package Neuropyxels (https://zenodo.org/records/5509776). We used the gen_sfc function with the default parameters to identify inhibitory putative monosynaptic connections using the Poisson Stark test for significance70 and a P-value threshold of 0.02. This procedure is based on convolving the cross-correlogram (computed with 0.5 ms bins) with a partially hollowed window. We searched for functional negative correlation in the cross-correlogram within 1 to 2.5 ms, from putative presynaptic neuron spike times. We then filtered obtained connections to identify the ones with a negative correlation peak within 2.5 ms in the z-scored correlogram, and a minimum z-scored correlation amplitude less than −10. z-Scored correlations (presynaptic to postsynaptic) were then visualized for each single connected pair in Fig. 5a and Extended Data Figs. 8b and 9a. The location of all connected pairs of neurons was also visualized in CCF space as described in the ‘Anatomical reconstructions and data analysis’ section above. To compute noise correlations between pre- and postsynaptic neuron firing during behaviour, we correlated spike rate in 20 ms bins across a window of 400 ms centred around the relevant behavioural timestamp.
Onset latency of neuronal activity before reach start
For each dataset (SNr, Midbrain, latRM), we analysed the activity of single neurons across all isolated reach start timestamps. Taking a timeframe of 0.5 s before reach start across trials, we identified neurons that were significantly (P < 0.001) modulated during any 20 ms window sliding 1 ms at a time, as compared to the baseline firing rate from −0.70 to −0.50 s before reach start (see modulation computation above). Having identified these neurons and the earliest modulated window before reaching start, we rolled back to find the earliest time window before a modulation P value of less than 0.05 was displayed. The start time of that 20 ms window for each neuron was considered to be the onset of a consistent neuronal activity change preceding reach onset across trials (Fig. 5b and Extended Data Fig. 8c). The distribution of onsets across neurons belonging to different brain regions or modulated positively and negatively for SNr was determined using a Kolmogorov–Smirnov test (Fig. 5c). We then plotted the z-scored average firing rate of single neurons detected as described above aligned to reach start (Fig. 5d and Extended Data Fig. 8c).
Software and statistics
All analyses were performed using custom code in Python or MATLAB as specified above. Sample sizes were not predetermined, and nonparametric statistical tests (Wilcoxon signed-rank and Kolmogorov–Smirnov tests) were always used to avoid assuming normal distributions. Probability densities were estimated using kernel density estimation with a Gaussian kernel. All P values are indicated either in the text or in the figure legends. Figures were prepared in CorelDRAW v.24.4. Mouse drawings were provided by E. Tyler and L. Kravitz through the SciDraw repository (www.scidraw.io) and adapted in CorelDraw.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.