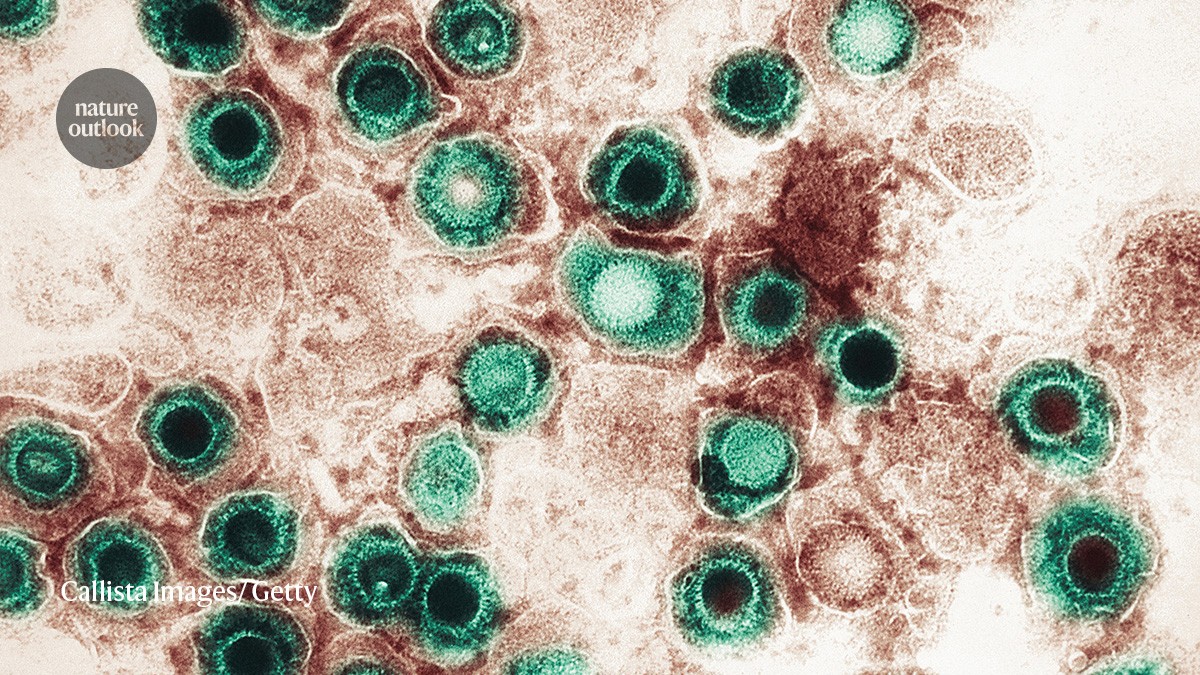
Herpesvirus might have a role in Alzheimer’s disease.Credit: Callista Images/ Getty Images
Much of Maria Nagel’s work as a neurologist at the University of Colorado in Aurora has focused on understanding the consequences of viral infection on the nervous system. Roughly a decade ago, she noticed an unusual pattern: people with previously stable dementia would sometimes see their condition deteriorate significantly after a bout of the viral infection shingles. “They had a rapid decline in a one-year period, and ended up in a nursing home,” she says.
Nature Outlook: Alzheimer’s disease
Shingles arises after the reactivation of the varicella zoster virus (VZV), which typically causes chickenpox early in life before becoming dormant for many decades. It is characterized by extremely painful rashes, but a growing number of clinicians and researchers have been uncovering evidence that VZV might also fuel the progression of Alzheimer’s disease and other forms of dementia.
Nagel and others are focusing on the potential involvement of VZV and other herpesviruses, which slumber in most people’s cells and can trigger a damaging immune response if reawakened. But there is evidence tentatively linking numerous infectious agents to Alzheimer’s, and some researchers think that there could be a more generic mechanism at play — almost any pathogen that accesses the brain might shift Alzheimer’s into overdrive.
More and more Alzheimer’s researchers, including former sceptics, are opening up to the possibility that infection contributes to neurodegeneration. “I was mentally opposing that idea for a long time,” says Michael Heneka, a neuroinflammation specialist at the University of Luxembourg in Esch-sur-Alzette. Now, Heneka says, “I think it’s an interesting hypothesis that deserves more studies.”
An inflammatory hypothesis
Historically, the Alzheimer’s field has been less than welcoming to theories that do not centre around amyloid-β and tau, the proteins that accumulate to form the plaques and tangles that are the hallmarks of the disease. Heneka encountered this himself while exploring the potential contribution of inflammatory processes in the late 1990s. “You were just lucky when you weren’t beaten up by the amyloid-β or tau people if you would mention immunology,” he jokes.
Since then, he and others have made a compelling case for the association of Alzheimer’s with a strong inflammatory response that suppresses neuronal function and accelerates the formation of amyloid-β plaques and tau tangles. “Inflammatory mediators are present in the cerebrospinal fluid as soon as amyloid starts to accumulate in the brain,” says Gwenn Garden, a neurologist at the University of North Carolina at Chapel Hill. Some of this inflammation is a consequence of early plaque formation, which creates a feedback loop that accelerates further amyloid-β aggregation, but lifestyle and environmental factors might also create an inflammatory environment in the brain that favours the development of Alzheimer’s.
One such source of inflammation could be viral or bacterial infections. It has proved challenging, however, to establish connections to between inflammation and specific pathogens. Ruth Itzhaki, who studies neurovirology at the University of Oxford, UK, was an early proponent of a role for herpesvirus in Alzheimer’s disease, and focuses especially on herpes simplex virus 1 (HSV-1) — a virus carried by two-thirds of people under 50. “It stays in a latent state most of the time,” she says, but adds that “when there are events like immunosuppression or inflammation, it reactivates and then causes both direct viral damage and also [further] inflammation”.
In the 1990s and 2000s, Itzhaki and her colleagues demonstrated that amyloid-β plaques usually contain viral DNA from HSV-1. Now, in work with biomedical engineer David Kaplan at Tufts University in Medford, Massachusetts, Itzhaki has used 3D models of human neuronal cell cultures to identify triggers that reawaken HSV-1 and induce Alzheimer’s pathology. These triggers include active infection with VZV1 and repetitive concussive injury2 — a known risk factor for the disease.
Other pathogens that persist in the nervous system, such as cytomegalovirus and various bacteria, are also being investigated for their potential to speed up neurodegeneration. But this focus on latent pathogens might be too narrow. Rudolph Tanzi, an Alzheimer’s researcher at Harvard Medical School in Boston, Massachusetts, thinks that a more generalized response to infection could be the culprit.
In 2010, Tanzi and long-time collaborator Robert Moir demonstrated that amyloid-β exerts potent antimicrobial effects in vitro3. This led to the suggestion that the production of amyloid-β by neurons might be a defence mechanism against pathogens. In 2018, Moir, who died in 2019, and Tanzi demonstrated that herpesvirus infection induces the release and aggregation of amyloid-β both in cultured human brain cells and in mice4. “They start to form, eventually, a plaque that acts as an extracellular trap for the microbe,” explains Tanzi.
He thinks that younger people have the capacity to efficiently clear away these pathogen-laden amyloid-β cobwebs before they become problematic. But as we age, he says, this ability wanes, “and the infection drives the pathology: amyloid plaques, tau tangles and neuroinflammation”.
Faint fingerprints
Although cell culture and animal experiments make an intriguing case for a connection between infection and Alzheimer’s, researchers have struggled to acquire equally robust evidence in humans.
Over the past 20 years, numerous studies have looked for viral DNA in the brains of people with Alzheimer’s or other neurodegenerative disorders, or have tried to detect antibodies circulating in the blood of people with dementia that indicate exposure to or active infection with herpesvirus. A 2019 review evaluated nearly 60 clinical studies of 8 herpesviruses, but was unable to distinguish any clear patterns of association5. The literature was “messy”, says Charlotte Warren-Gash, an epidemiologist at the London School of Hygiene & Tropical Medicine who led the review. “Many studies were not very good quality — very small studies, studies that didn’t control for confounders, studies that used designs that I as an epidemiologist might not have selected,” she says. In 2022, her team set out to evaluate levels of circulating antibodies against HSV-1 in people with hereditary Alzheimer’s and found no meaningful connection6.
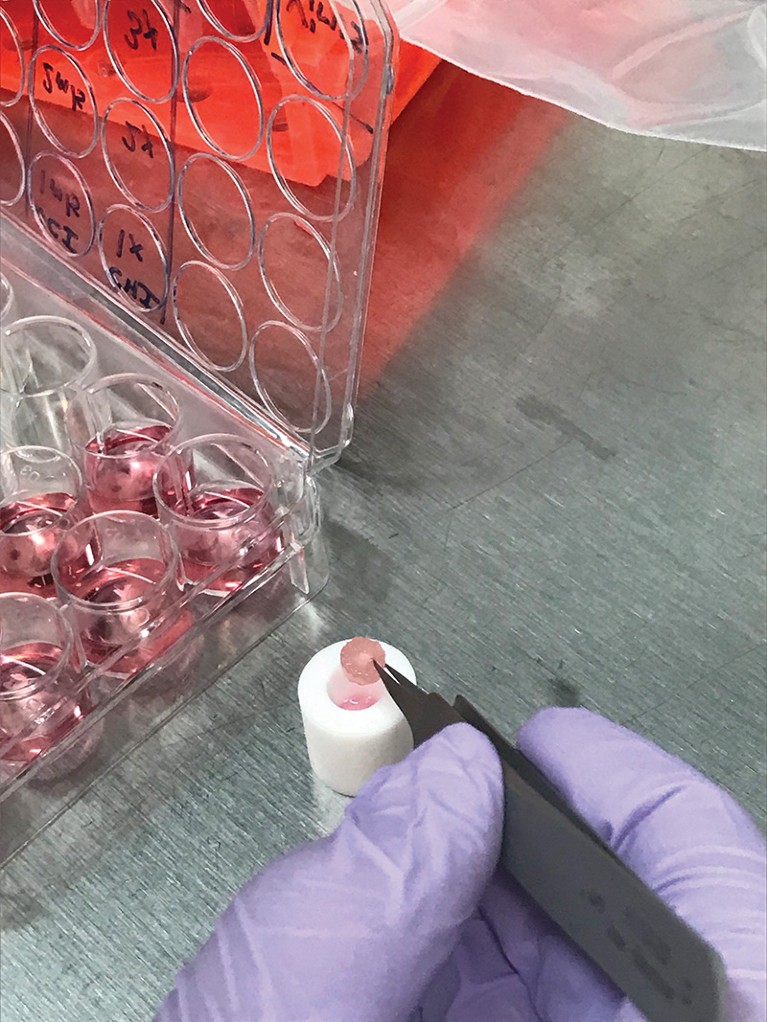
Tissue models are used to identify triggers that induce Alzheimer’s pathology.Credit: Dana Cairns
Some studies carried out since the review by Warren-Gash and her colleagues seem to offer supporting, but not conclusive, evidence for a link between active viral infection and Alzheimer’s. For example, Nagel was part of a 2023 study evaluating a large group of people at high risk of hereditary early-onset Alzheimer’s in Colombia that revealed physiological changes that are normally associated with the response to viral infection7. “We showed that familial Alzheimer’s patients actually had signatures of viral processes and infection in the olfactory bulb”, and more inflammation in the bundles of nerve fibres that run to these smell centres in the brain, compared with healthy individuals, says Nagel. The study did not, however, attempt to detect the viruses themselves.
In search of indirect evidence of a connection, some groups have explored whether people who are vaccinated against shingles — which thwarts the reactivation of VZV — are at a lower risk of dementia. Early studies that sought to establish such a connection were vulnerable to biases that made their results impossible to interpret confidently. “We know that people who are more health motivated, more interested in preventive health care, more socio-economically advantaged, are more likely to get vaccinated,” says Pascal Geldsetzer, an epidemiologist at Stanford University in California.
Geldsetzer has sought to overcome this bias with ‘natural’ experiments based on how the shingles vaccine was rolled out in various nations. A study8 from his team has evaluated electronic health-care records in England and Wales, where there was a birth-date-based cut off for eligibility to receive the vaccine when it was rolled out. “Just a week’s difference in age at the time of the start of the programme meant you had this huge difference in ever getting vaccinated,” says Geldsetzer. Otherwise, the demographics of these two cohorts were largely identical. The study found evidence of a protective effect, with a particularly notable reduction in risk of both diagnosis of dementia and death from dementia for women. The reduction in women was more than fivefold greater than that in men, in whom the observed protective effects were not statistically significant. Geldsetzer adds that the data indicate that there was a roughly 20% reduction in the odds of developing dementia in the seven-year follow-up period after receiving the shingles vaccination.
These findings are more suggestive than conclusive, however, and efforts to link vaccination to protection from Alzheimer’s can be confounded by numerous factors. Warren-Gash points out that dementia is often under-reported in health records — people with mild symptoms might be overlooked, which would skew the results of studies focused on dementia risk.
Intriguingly, Garden says that there is epidemiological evidence that vaccines against infectious diseases such as tuberculosis and influenza might also protect against dementia. But it is unclear whether this putative benefit stems from preventing these specific infections or from boosting immunity in general.
Tanzi favours the latter hypothesis. “Every time you have a vaccine, you amp up the peripheral immune system and monocytes get into the brain and help clear amyloid,” he says, referring to a subset of immune cells that help to fend off infectious agents and eliminate damaged cells.
The frequent absence of a concrete diagnosis in medical records is a challenge to various attempts to formulate a link between infection and Alzheimer’s disease. Accordingly, Geldsetzer’s studies have focused on dementia in general rather than Alzheimer’s specifically. “It’s not just that they’re poorly recorded, it’s that often they’re not easy to diagnose at all,” says Geldsetzer. As a result, it might well turn out that vaccination has broader neuroprotective benefits.
In fact, the hunt for an infectious link goes beyond Alzheimer’s. Several studies have now suggested that a range of neurodegenerative diseases — including multiple forms of dementia — are accelerated by viral infection. For example, a 2023 study led by scientists at the US National Institutes of Health evaluated data from hundreds of thousands of individuals in Finland and the United Kingdom and identified dozens of putative associations between infection with viruses, including VZV and influenza, and the subsequent onset of neurological disorders9.
Best practices
But resolving the role of infection in Alzheimer’s disease will require more sophisticated analysis than is currently the norm. Herpesviruses pose a particular challenge. Whereas reactivated VZV causes shingles, most other awakened herpesviruses are either asymptomatic or mildly symptomatic. Cold sores appear, for example, when HSV-1 reactivates. If symptoms are not sufficiently severe to drive a person to seek medical care, it is impossible to retroactively establish a link between a diagnosed, active infection and the eventual onset of dementia.
Measuring antiviral antibodies circulating in the blood is also less than ideal, because they could be the result of viral reactivation anywhere in the body, not just the brain. And direct detection of viruses in brain-tissue samples is extremely difficult because it requires ultrasensitive molecular methods. “A lot of the push back against this research is coming from issues with the validity of the techniques for detecting viral particles in autopsy specimens,” says Garden.
Nagel aspires to track the interplay between infection and dementia over long periods of time in people with confirmed infections through routine imaging, molecular profiling and cognitive testing. “I think that’s the best way to do it,” she says. But this won’t be cheap: she has calculated that a small study of just 60 people could cost up to US$7 million a year. Performing smaller but similar studies with non-human primates would be cheaper, but even these could still cost several hundred thousand dollars for just a few animals.
In 2023, an international group of researchers came together as part of the Alzheimer’s Pathobiome Initiative (AlzPI) to draw up a road map for conducting more systematic and reproducible research into the contribution of microorganisms to the disease10. This is becoming especially important as researchers continue to tentatively link more microbes to Alzheimer’s. For example, several studies have identified putative connections between dementia and periodontal-disease-causing pathogens such as the bacterium Porphyromonas gingivalis11. But once again, proving causality remains difficult; Tanzi points out that poorer oral hygiene is common in people experiencing dementia, creating conditions that allow these bacteria to proliferate in a person’s mouth and potentially make their way to the brain.
Tanzi thinks that the community should focus on systematically generating sequencing and other molecular data from as many fresh brain specimens as possible — a top priority for his own laboratory. But he is also increasingly of the view that these studies will not decisively reveal a clear ‘hit’, but rather point to a general trend of infection, tipping the balance towards Alzheimer’s by creating conditions that favour amyloid-β build-up in the brain. “There’s not just one smoking gun — there’s a smoking gun range,” he says. In that scenario, an overly narrow focus on the specific role of one or two viruses runs the risk of not seeing the forest for the trees.
More from Nature Outlooks
A more inclusive search that focuses on patterns of infection and the associated immunological and neurological responses could therefore yield ironclad evidence of a link between active infection and subsequent neurodegeneration. Warren-Gash is currently pursuing such a survey with a study that aims to broadly evaluate signatures of bacterial or viral infection in serum samples collected from 50,000 individuals as part of the UK Biobank initiative. “I don’t think that there’s going to be one pathogen that just sticks out — it’s more about the cumulative burden,” she says.
This is in keeping with a broader pattern of findings indicating that the systemic effects of infections outside the brain, ranging from pneumonia to sepsis, can contribute to dementia. A study of nearly one million people, conducted by researchers including Warren-Gash, found that the risk of dementia increased by 50% over a median follow-up period of roughly five years after contracting various common infections. If the infection led to hospitalization, the risk was even greater12. For now, investigators are struggling to disentangle whether these observations are a product of body-wide inflammation, a direct response to microbes present in the brain, or simply the result of greater overall frailty in older individuals fending off infection.
As evidence accumulates, researchers are increasingly open to the idea that even transient infections can have long-lasting effects on cognition and mental function. For example, Garden cites ongoing research into long COVID after infection with SARS-CoV-2, and chronic fatigue syndrome, also known as myalgic encephalitis, which is thought to arise in the aftermath of poorly resolved viral infections. But given the Alzheimer’s field’s historical focus on framing disease pathology mainly in amyloid-centric terms, she also recognizes that steering the community towards a more complicated and heterogeneous set of potential disease processes will be an uphill struggle. “It’s very difficult to interpret the results when you look at more than one thing at a time,” says Garden. “That’s a big part of the problem in trying to sort something like this out.”