Binder design using sequence input RFdiffusion
For each target, approximately 10,000–50,000 diffused designs were generated given only sequence input of the target using RFdiffusion (v1.1.0). The resulting library of backbones were sequence designed using ProteinMPNN (v1.0.1)24, followed by AF2 + initial guess26. Initial guess is the protocol in which the protein structure provided to the model as an initial guess is first converted to AlphaFold atom positions. These positions are then provided, along with the standard model inputs into the AlphaFold Model Runner. In the AlphaFold class of the AlphaFold code, on the first recycle, the prev_pos variable is initialized to the input AlphaFold atom positions as opposed to the standard initialization of all zeros26. The resulting designs were filtered based on interface pAE_interaction, pLDDT. The pAE of interaction (pAE_interaction) between the binder and the target was used to evaluate the confidence of the predicted interface. Lower pAE_interaction values indicate higher confidence in the relative positioning of the two proteins. The per-residue pLDDT score measures the confidence of AF2 in the local structure of the binder. In addition, AF2 monomer was performed using only the binder sequence to filter based on the monomer pLDDT of the binder and RMSD to the binder design model (Supplementary Table 2). Subsequently, FastRelax was executed to obtain Rosetta metrics52. The resulting binders were then further filtered based on criteria including contact_molecular_surface53, ddG54, SAP score55 and the numbers of hydrogen bonds. Specific filtering criteria were carefully selected to narrow down the set to 48–96 designs for each target.
Two-sided partial diffusion to optimize binders
Partial diffusion enables the input structure to be noised only up to a user-specified timestep instead of completing the full noising schedule. The starting point of the denoising trajectory is therefore not a random distribution. Rather, it contains information about the input distribution resulting in denoised structures that are structurally similar to the input. Unlike one-sided directional partial diffusion, which solely diversifies the conformation of the binder while keeping the target fixed, two-sided partial diffusion allows simultaneous conformational changes in both the target and the binder. The input designs were subjected to 5–25 noising timesteps out of a total of 50 timesteps in the noising schedule, and subsequently denoised. Approximately 5–50,000 partially diffused designs were generated for each target. The resulting library of backbones were sequence designed using ProteinMPNN24, followed by AF2 + initial guess26. The resulting designs were filtered in the same way as the designs from the aforementioned sequence input diffusion process.
Secondary structure specifications
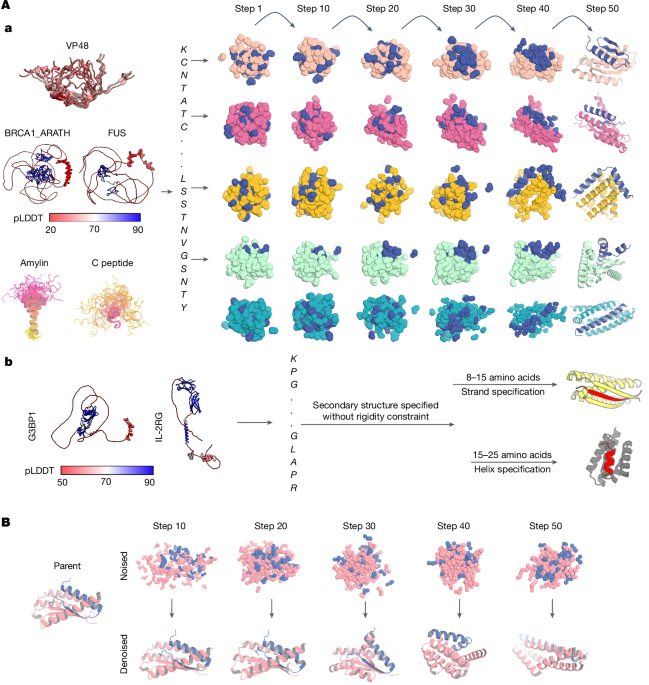
To permit specification of the secondary structure (but not three-dimensional coordinates) of the target, a modified version of RFdiffusion was trained that permits specification of the secondary structure of a region, along with its sequence. The training strategy largely followed that used to train previous RFdiffusion models5,12, with some modifications. A summary is provided below.
For an overview of ‘base’ RFdiffusion training, Rfdiffusion5 is a denoising diffusion probabilistic model, which is fine-tuned from the RoseTTAFold structure prediction model25,56. In RFdiffusion, the N-Ca-C frame representation (translation and orientation) of protein backbones25,57 is used, and, over 200 discrete timesteps, these backbone frames are corrupted following a defined forwards noising process that noises these frames to distributions indistinguishable from random distributions (three-dimensional Gaussian distribution for translations and uniform SO(3) distribution for rotations). RFdiffusion is trained to reverse this noising process, predicting the true (X0) protein structure at each timestep of prediction (starting from randomly sampled translations and rotations). Successive predictions are used to ‘self-condition’ predictions through an inference trajectory, and mean squared error losses minimize the error between forwards and reverse processes. Full details of training are described in Watson et al.5.
For modifications to permit secondary structure specification of the target, as in the original RFdiffusion fine-tuned for protein binder design, RFdiffusion was trained 50% of the time on single chains from the PDB < 384 amino acids in length, and 50% on heterocomplexes. In the latter case, one chain (less than 250 amino acids in length) was designated the ‘binder’, and when necessary the other ‘target’ chain was radially cropped around the interface (to 384, which is the length of the binder residues). For single-chain examples, 20% of the time, the whole backbone was noised; in the other 80% of cases, 20–100% of the protein backbone was noised. For heterocomplex examples, the whole binder chain was noised. In addition, and in contrast to the original RFdiffusion model trained for protein binder design, up to 50% of the noised monomer structure had sequence provided in the noised region. For heterocomplexes, up to 50% of the target chain backbone was also noised, whereas its sequence was provided to RFdiffusion. This permits RFdiffusion to condition on the sequence of the target chain in the absence of three-dimensional structure.
To permit specification of the secondary structure of the target (when three-dimensional coordinates are not provided), secondary structure and ‘block adjacency’5 information were provided to RFdiffusion in exactly the manner described in Watson et al.5. In brief, 50% of the time, RFdiffusion was provided with a (partially masked; 0–75%) secondary structure of the example protein chain or heterocomplex, and (an independently sampled) 50% of the time a (partially masked; 0–75%) block adjacency of the protein chain or heterocomplex. In addition, 50% of the time, the whole interchain block adjacency was masked in heterocomplex examples. This permits RFdiffusion to condition on a (partially) pre-specified secondary structure (and/or adjacency information) of the target. This version of RFdiffusion was trained for seven epochs.
To design binders using RFdiffusion through secondary structure specification, for each target, approximately 10,000 diffused designs were generated through sequence input of the target with the additional secondary structure specification. The resulting library of backbones were sequence designed using ProteinMPNN24, followed by AF2 + initial guess26. The resulting designs were filtered in the same way as the designs from the aforementioned sequence input diffusion process.
Backbone extension for VP48 binder design
During the design campaign, not all designs provided sufficient interactions to the whole sequence of the target, especially the loopy regions. To explore and guide RFdiffusion to make more interactions around certain regions, we selected 20 AF2 passing designed complexes from the round one design campaign, based on the above criteria and manual selection. For each base design, we requested RFdiffusion to extend the binder backbone with 10–20 amino acids from either N terminus, C terminus or both (depending on where the loopy region was located). This was done with the inpaint flavour published in the original RFdiffusion work5. Two thousand designs were performed each run, followed by the same MPNN and AF2 predictions as above.
Computational filtering
Precise metrics cut-offs changed for each design campaign to get to an orderable set, but largely focused on interface pAE_interaction < 10, pLDDT > 90, number of hydrogen bonds > 11, RMSD < 0.5, sap score < 45 and Rosetta ddG < −40 (ref. 26).
Computational time and hardware usage
A typical binder design task, generating an approximately 80–150 residue binder, each backbone design using RFdiffusion took approximately 25–30 s when run on a single NVIDIA RTX2080 or A4000 GPU, using one CPU core and approximately 8 GB of RAM. The subsequent sequence design step using ProteinMPNN was notably faster and less resource intensive, requiring less than 0.5 s per backbone on a standard CPU (for example, Intel Xeon E5-2680).
Gene construction of designed binders
The designed protein sequences were optimized for expression in Escherichia coli. Linear DNA fragments (eBlocks, Integrated DNA Technologies) encoding design sequences included overhangs suitable for Golden Gate cloning into the LM670 vector (Addgene #191552) for protein expression in E. coli. LM670 is a modified expression vector containing a kanamycin resistance gene, a ccdB lethal gene between BsaI cut sites and a C-terminal hexahistidine, commonly referred to as His tag.
Binding screening
For screening for all designs except the ones of partial diffusion design for amylin-68n (Fig. 2a), the designs were screened by BLI (method details described below). Linear gene fragments encoding binder design sequences were cloned into LM670 using Golden Gate assembly. Golden Gate subcloning reactions of binders were constructed in 96-well PCR plates in 4 µl volume. One microlitre reaction mixtures were then transformed into a chemically competent expression strain (BL21 (DE3)). After 1-h recovery in 100 µl SOC medium, the transformed cell suspensions were directly transferred into a 96-deep-well plate containing 900 µl of LB media with kanamycin. After overnight incubation in 37 °C, 100 μl of growth culture was inoculated into 96-deep-well plates containing 900 µl of auto-induction media (autoclaved TBII media supplemented with kanamycin, 2 mM MgSO4, 1 × 5,052). After overnight incubation (6 h at 37 °C followed by additional 18 h at 30 °C), cells were harvested by centrifugation (15 min at 4,000g). Bacteria were lysed for 15 min in 200 μl lysis buffer (1× BugBuster (70921-4, Millipore), 0.01 mg ml−1 DNAse, and 1 tablet of Pierce protease inhibitor tablet per 50 ml culture). Lysates were clarified by centrifugation at 4,000g for 10 min, before purification on Ni-charged MagBeads (L00295, Genscript; wash buffer (25 mM Tris pH 8.0, 300 mM NaCl and 30 mM imidazole) and elution buffer (25 mM Tris pH 8.0, 300 mM NaCl and 400 mM imidazole)). Subsequently, the elutions were directly subjected to a BLI test and the final concentration was approximately 1 μM. The designs exhibiting binding signals were subsequently analysed by BLI through titration.
For amylin-68n, the designs from partial diffusion were expressed and purified using the same way as mentioned above. In addition to the designs, plasmids expressing target peptide fused with sfGFP (no His tag) were transformed into BL21 (DE3) cells, and overnight outgrowths were cultured in 5 ml of LB media with kanamycin. After overnight incubation in 37 °C and 250 rpm, growth cultures were inoculated into 50 ml auto-induction media. After overnight incubation in 37 °C and 250 rpm, cells were harvested by centrifugation (15 min at 4,000g), then resuspended in 20 ml lysis buffer (25 mM Tris-HCl, 150 mM NaCl, 0.1 mg ml−1 lysozyme, 10 μg ml−1 DNAse I and 1 mM PMSF). Of lysate of each binder, 100 µl was mixed with 100 µl of lysate of target peptide fused with sfGFP and incubated at room temperature for 15 min for co-lysis and target binding to the binders. Mixed lysates were applied directly to a 100 µl bed of Ni-NTA agarose resin in a 96-well fritted plate equilibrated with a Tris wash buffer. After sample application and flow through, the resin was thoroughly washed, and samples were eluted in 200 µl of a Tris elution buffer containing 300 mM imidazole. All eluates were sterile filtered with a 96-well 0.22-µm filter plate (203940-100, Agilent) before size-exclusion chromatography. Protein binders were then analysed for target binding via sfGFP co-elution with the His-tagged binder. High-performance liquid chromatography (HPLC) analyses were conducted using an Agilent HPLC system (Agilent 1260 Infinity II Liquid Chromatography system). Co-lysates were run on a Superdex200 Increase 5/150 GL column (28990945, Cytiva) with buffer of 25 mM Tris-HCl and 150 mM NaCl. To assess the binding interaction between the target and the binder, we monitored the elution profile of sfGFP using an absorbance wavelength of 395 nm, alongside a simultaneous measurement at 280 nm for total protein content to determine the extent of overlap between 395 nm and 280 nm, which indicates the binding interaction.
Medium-scale protein expression and purification
For further validation, the initial hits were expressed at the 50-ml scale via auto-induction for approximately 24 h, in which the first 6-h cultures were grown at 37 °C and the remaining time at 22 °C. Cultures were harvested at 4,000g for 10 min and resuspended in approximately 20 ml lysis buffer (25 mM Tris-HCl, 150 mM NaCl, 0.1 mg ml−1 lysozyme, 0.01 mg ml−1 DNAse, 1 mM PMSF and 1 tablet of Pierce protease inhibitor tablet per 50 ml culture). Sonication was performed with a four-prong head for 5 min total, 10-s pulse on–off at 80% amplitude. The resulting lysate was clarified by centrifugation at 14,000g for 30 min. Lysate supernatants were applied directly to a 1-ml bed of Ni-NTA agarose resin equilibrated. After sample application and flow through, the resin was thoroughly washed, and samples were eluted by an elution buffer containing 400 mM imidazole. After elution, protein samples were filtered and injected into an autosampler-equipped Akta pure system on a Superdex S75 Increase 10/300 GL column at room temperature. The size-exclusion chromatography running buffer was 25 mM Tris-HCl and 150 mM NaCl pH 8. Protein concentrations were determined by absorbance at 280 nm using a NanoDrop spectrophotometer (Thermo Scientific) using their extinction coefficients and molecular weights obtained from their amino acid sequences.
BLI binding experiments
BLI experiments were performed on an Octet Red96 (ForteBio) instrument, with streptavidin-coated tips (18-5019, Sartorius). Buffer comprised 1X HBS-EP+ buffer (BR100669, Cytiva) supplemented with 0.1% w/v bovine serum albumin. Before target loading, each design was tested for binding against unloaded tips. Of biotinylated target protein, 50 nM was loaded on the tips for 50 s followed by a 60-s baseline measurement. After loading, all designs underwent a 60-s baseline, 300–1,200-s association and 200–800-s dissociation. Baseline measurements of unloaded tips were subtracted from their matched measurement of the loaded tip. The hits were taken forwards for further titration experiments, for which concentration, association and dissociation times were chosen based on apparent affinity from the single-point screen. Global kinetic fitting was used to determine Kd across the dilution series.
In the specificity test of the designed binders, final concentrations were 2, 0.667 and 0.222 μM for most binders; 0.833, 0.277 and 0.093 μM for VP48; and 5, 2 and 0.555 μM for BRCA1_ARATH-35 and FUS-40.
Circular dichroism experiments
For circular dichroism experiments, designs were diluted to 0.4 mg ml−1 in 25 mM Tris-HCl and 150 mM NaCl. Spectra were acquired on a JASCO J-1500 circular dichroism spectrophotometer. Thermal melt analyses were performed between 25 °C and 95 °C, measuring circular dichroism at 222 nm. All reported measurements were acquired within the linear range of the instrument.
Affinity enrichment of amylin analysed by LC–MS/MS
Bead preparation
Anti-amylin binder-coated beads were prepared by conjugating each amylin-targeted binder (amylin-68n) to paramagnetic M280 tosylactivated beads (Invitrogen). Each sample reaction conjugated 1 µg of binder to 225 µg of beads. Beads were blocked with a solution of 0.01% bovine serum albumin (BSA) in 0.2 M Tris to minimize nonspecific interactions. An off-target binder-conjugated bead was included for quantification of nonspecific binding. A BSA-blocked bead without a bound binder was used as a negative control, and an anti-GPVGPSGPPGK (GPVG) peptide monoclonal antibody-conjugated bead was used as a positive control for the affinity binding step.
Sample preparation
Human amylin peptide (non-amidated) was purchased from Anaspec and reconstituted to 2 mg ml−1 in dimethylsulfoxide (DMSO). A secondary peptide stock (diluted into 50 µM in 5% acetonitrile, 0.1% formic acid and 0.01% BSA in water) was reduced with dithiothreitol (10 mM final concentration) and alkylated with iodoacetamide (30 mM final concentration). Excess iodoacetamide was quenched with additional dithiothreitol (5 mM final added concentration). This solution was diluted to a working stock of 10 μM with dilution solvent. Aliquots of the working stock were made in 1.5-ml LoBind tubes and stored at −20 °C to avoid repeated freeze–thaw cycles.
Human specimens
Human plasma samples were composed of pooled de-identified leftover clinical samples obtained from the clinical laboratories at the University of Washington Medical Center. The use of de-identified leftover clinical samples was reviewed by the University of Washington Human Subjects Division (STUDY00013706).
Affinity enrichment
Amylin capture experiments were performed using three types of coupled beads (amlin-68n, an off-target binder and BSA blocked) in PBS containing 0.1% CHAPS as well as pooled normal human EDTA-anticoagulated plasma.
Samples were prepared by spiking the working stock of alkylated amylin to a final concentration of 20 nM in 100 µl of either PBS–CHAPS or pooled plasma. Additional PBS–CHAPS was added to each sample, followed by coupled beads. GPVG peptide and anti-GPVG monoclonal antibody-conjugated beads were added to each sample as a positive control. The mixtures were shaken for 1 h at 900 rpm and room temperature (Thermomixer, Eppendorf). The supernatant was removed and the beads were washed twice with 200 μl of PBS–CHAPS. Bound peptides were eluted in 50 µl of elution solvent (20% acetic acid, 10% acetonitrile, 10% DMSO and 0.001% BSA in water) with shaking for 8 min (900 rpm at room temperature). Each bead type (two anti-amylin binders, one off-target binder and one BSA blocked) was assessed in separate samples and each was prepared in triplicate.
Sample analysis was performed by LC–MS/MS using a Shimadzu Nexera LC-XR HPLC coupled to a Sciex 6500+ triple quadrupole tandem mass spectrometer in multiple reaction monitoring mode. Specifications for the liquid chromatography, mass spectrometer and multiple reaction monitoring methods are included in Supplementary Tables 3–5.
Data analysis
Data processing was performed with Skyline Daily (v23.1.1.459). Chromatographic peak area was calculated by summing the peak area of all transitions for each peptide. The chromatographic peak areas observed during blank (elution solvent) injections were subtracted as background from sample peak areas before performing further data reduction. The signal from BSA and GPVG beads was for quality control of the assay and evaluated before processing of the experimental data.
Seven types of samples were analysed:
-
(1)
Group A: alkylated amylin peptide spiked directly into elution solvent served as the reference peak area for 100% recovery of amylin peptide.
-
(2)
Group B: paramagnetic tosyl-activated beads conjugated to an off-target binder were incubated in PBS–CHAPS spiked with alkylated amylin. The peak area of this negative control was used to quantify nonspecific binding.
-
(3)
Group C: amylin-targeted binders conjugated to paramagnetic tosyl-activated beads were incubated in PBS–CHAPS spiked with alkylated amylin. The peak areas of these samples were used to quantify the percent recovery of amylin by affinity enrichment.
-
(4)
Group D: an off-target binder conjugated to paramagnetic tosyl-activated beads was incubated with unspiked plasma. The peak area of this negative control was used to quantify the nonspecific signal from beads binding to plasma components.
-
(5)
Group E: amylin-targeted binders conjugated to paramagnetic tosyl-activated beads were incubated with unspiked plasma. The peak areas observed in these samples were used to quantify the nonspecific signal from the binders binding to plasma components (that is, assuming no non-amidated amylin in normal plasma).
-
(6)
Group F: an off-target binder conjugated to paramagnetic tosyl-activated beads was incubated with spiked plasma. The peak area of this negative control was used to quantify nonspecific binding.
-
(7)
Group G: amylin-targeted binders conjugated to paramagnetic tosyl-activated beads were incubated with spiked plasma. The peak areas of these samples were used to quantify percent recovery of amylin by affinity enrichment.
The percent recovery of each binder-coated bead type was calculated using equation (1) provided in Supplementary Information. The percent recovery of each binder-coated bead type was analysed using Graph Pad Prism 8.
Preparation of SSM libraries
Saturation mutagenesis (SSM) was performed on all designs to gain a better understanding of the peptide-binding modes. CP-35 was selected for detailed analysis due to its structural complexity and the high-quality SSM data obtained. For CP-35, we ordered a SSM library covering all the 159 amino acids. The chip-synthesized DNA oligos for the SSM library were then amplified and transformed to EBY100 yeast together with a linearized pETCON3 vector. Each SSM library was subjected to an expression sort first, in which the low-quality sequences due to chip-synthesizing defects or recombination errors were filtered out. The collected yeast population, which successfully expresses the designed mutants, were regrown and subjected to the next round of peptide-binding sorts. Two rounds of with-avidity sorts were applied at 1 μM concentration of CP followed by one round of without-avidity sorts with CP concentrations at 200 nM, 40 nM, 8 nM, 1.6 nM and 0.32 nM. The peptide-bound yeast populations were collected and sequenced using the Illumina NextSeq kit. The mutants were identified and compared with the mutants in the expression libraries. Enrichment analysis was used to identify beneficial mutants and provide information for interpreting the peptide-binding modes. For each mutant, the fraction of cells collected in each of the five titration sorts of decreasing concentration was measured. The sorting concentration 50, the concentration at which 50% of the expressing cells are collected, was calculated and plotted in heatmaps for SSM analysis.
X-ray crystallography
We attempted to solve structures for all of our designs, but only the amylin and G3BP1 complexes successfully crystallized.
Crystallization experiments were conducted using the sitting drop vapour diffusion method.
Initial crystallization trials were set up in 200-nl drops using the 96-well plate format at 20 °C.
Crystallization plates were set up using a Mosquito LCP from SPT Labtech, then imaged using UVEX microscopes and UVEX PS-256 from JAN Scientific. Diffraction quality crystals formed in 0.1 M succinic acid, sodium phosphate monobasic monohydrate, glycine mixture at pH 6 and 30% w/v PEG 1000 for amylin-22. For G3BP1-11, diffraction quality crystals appeared in 0.05 M calcium chloride dihydrate, 0.1 M Bis-Tris pH 6.5, and 30% v/v polyethylene glycol monomethyl ether 550. For amylin-18αβ, diffraction quality crystals appeared in 3.2 M ammonium sulfate and 0.1 M citrate pH 5.0.
Diffraction data were collected at the National Synchrotron Light Source II on beamline 17-ID-1 (AMF) for amylin-18αβ and amylin-22αβL. Diffraction data were collected at the Advanced Light Source beamline 821 for G3BP1-11. X-ray intensities and data reduction were evaluated and integrated using XDS42 and merged/scaled using Pointless/Aimless in the CCP4 program suite43. Structure determination and refinement starting phases were obtained by molecular replacement using Phaser (v2.5.0)44 using the designed model for the structures. Following molecular replacement, the models were improved using phenix.autobuild, with rebuild-in-place to false and using simulated annealing. Structures were refined in Phenix (v1.21.1_5286)45. Model building was performed using Coot (v0.9.8.7)46. The final model was evaluated using Molprobity (v4.5.2)47. Data collection and refinement statistics have been recorded in Extended Data Table 1. Data deposition, atomic coordinates and structure factors reported in this paper have been deposited in the PDB (http://www.rcsb.org/) with the accession codes 9CC5, 9CC6 and 9NZH, respectively.
We used PyMOL (v2.4.0) and UCSF Chimera (v1.14) for generating figures.
Cell culture
HeLa cells (from the American Type Culture Collection) were cultured in DMEM (11965-092, Gibco) at 37 °C in a humidified atmosphere containing 5% CO2, supplemented with 10% (v/v) FetalClone II serum (SH3006603, Cytiva) and 1% penicillin–streptomycin (15140122, Thermo Fisher).
CRISPR–Cas9 knockout of IL2RG
Pooled IL2RG-knockout HeLa cells were generated using the Gene Knockout kit V2 from Synthego, using multi-guide single guide RNA targeting IL-2RG (guide 1: CAUACCAAUAAUGCAGAGUG guide 2: UCGAGUACAUGAAUUGCACU and guide 3: GAAACACUGAGGGAGUCAGU). The ribonucleoprotein complex with a ratio of 4.5:1 of single guide RNA and Cas9 was delivered following the protocol of the SE Cell Line 4D-Nucleofector X Kit S (V4XC-1032, Lonza), using the nucleofection program CN-114 on the Lonza 4D X unit.
Transient transfection
Plasmids for binder–mScarlet, IL-2RG–eGFP, and other target-eGFP-mito-tag were either synthesized and cloned by Genscript or constructed in-house. HeLa cells were seeded at 70–80% confluency in a chambered coverslip with 18 wells (81816, ibidi). At the same time, HeLa cells were reverse transfected using Lipofectamine 3000 transfection reagent (L3000008, Thermo Fisher) according to the manufacturer’s protocol.
Fluorescence imaging
Four-colour, 3D images were acquired with a commercial OMX-SR system (GE Healthcare). Toptica diode lasers with excitation at 488 nm and 568 nm were used. Emission was collected on three separate PCO.edge sCMOS cameras using an Olympus ×60 1.42 NA plan apochromat oil immersion lens. Images (512 × 512; pixel size of 6.5 μm) were captured with no binning. Acquisition was controlled with AcquireSR Acquisition control software. Z-stacks were collected with a step size of 250 nm. Images were deconvolved in SoftWoRx 7.0.0 (GE Healthcare) using the ratio method and 200-nm noise filtering. Images from different colour channels were registered in SoftWoRx using parameters generated from a gold grid registration slide (GE Healthcare).
For imaging stress granules, cells were washed twice with FluoroBrite DMEM imaging media and subsequently imaged in the same media in the dark at room temperature. Epifluorescence imaging was performed on a Yokogawa CSU-X1 spinning dish confocal microscope with either a Lumencor Celesta light engine with seven laser lines (408, 445, 473, 518, 545, 635 and 750 nm) or a Nikon LUN-F XL laser launch with four solid-state lasers (405, 488, 561 and 640 nm), ×40/0.95 NA objective or ×60/1.4 NA oil immersion objective and a Hamamatsu ORCA-Fusion scientific CMOS camera, both controlled by NIS Elements 5.30 software (Nikon). The following laser and filter combinations (centre/bandwidth) were used: excitation of 473 nm and emission of 525/36 nm for GFP, and excitation of 545 nm and emission of 605/52 nm for RFP. Exposure times were 500 ms for all channels, with no emission gain set and no neutral density filter added. All epifluorescence experiments were subsequently analysed using ImageJ (v1.54p). Brightfield images were acquired on the ZOE Fluorescent Cell Imager (Bio-Rad).
ThT fluorescence assay
Amylin fibrils at various growth stages (0, 3 and 24 h) were adequately mixed with ThT at a molar ratio of 1:1 and added into 96-well-plates containing different types and concentrations of binders (amylin-75, amylin-36, amylin-22 and amylin-68n). The samples were then incubated at 37 °C for 1–18 h with 600 rpm orbital shaking. ThT fluorescence signals were measured using a Thermo Varioskan Flash Multi Detection Microplate Reader (0 and 3 h) or a Perkin Elmer EnSight Multifunctional Microplate Reader (24 h) with excitation wavelength at 440 nm and an emission wavelength at 482 nm.
NS-EM experiment
Samples for NS-EM were dropped onto freshly glow-discharged carbon-coated copper grids and incubated for 1 min, and excess sample was removed by blotting on filter paper. The grids were then stained with 2% (w/v) uranyl acetate for 1 min, and excess uranyl acetate was blotted off. Finally, the grids were examined using a Tecnai Spirit transmission electron microscope (FEI) at an acceleration voltage of 120 kV.
Lysosomal trafficking of amylin monomers and fibrils
HEP3B cells (obtained from the American Type Culture Collection) were plated onto eight-well Labtek slides overnight. On the day of the treatment, biotinylated amylin monomers or fibrils were complexed with Alexa Fluor 647-labelled streptavidin (Thermo Fisher) at 500 nM. These were then pre-complexed with amylin-36 or amylin-36-EndoTags, and cells were treated with this complex for 20 h. Cells were then fixed with 4% paraformaldehyde, permeabilized and stained with LAMP1 monoclonal antibody (H4A3), Alexa Fluor 488 (MA5-18121, Thermo Fisher; 1:200 dilution), followed by goat anti-mouse IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 488 (A-11001, Thermo Fisher; 1:500 dilution) and counterstained with DAPI. Cells were washed with DPBS and imaged with a Nikon A1R confocal microscope using a Plan Fluor ×60, 1.30 NA oil objective. The following laser settings were used: 405-nm violet laser, 488-nm blue laser and 639-nm red laser. Quantification of internalized amylin fibrils (left) and monomers (right) by flow cytometry was analysed using Graph Pad Prism 8. Flow cytometry was performed in Attune NxT flow cytometer (Thermo Fisher). The data were analysed in FlowJo (v9) software. The FACS sequential gating/sorting strategy is shown in Supplementary Fig. 1.
PhaseScan
Droplet microfluidic experiments using PhaseScan58 were perfomed as previously described59,60.
Experiments were conducted under physiological conditions with 150 mM KCl and 50 mM Tris-HCl at pH 7.4. In all cases, 2% w/v PEG 10 K (Thermo Fisher Scientific) was added to the solutions. A 10 µM solution of G3BP1-emerald in physiological buffer was prepared by diluting the protein from a stock solution in 1 M KCl and 50 mM Tris pH 7.4. In addition, a stock solution of the binder G3BP1-11 was prepared at 20 µM in physiological buffer. A 200 ng µl−1 polyA RNA (Merck) solution, labelled with 3 µM Alexa Fluor 647 for concentration measurement, was also prepared. For the microfluidic experiment, four aqueous solutions containing protein, binder, RNA and buffer, along with an oil solution for droplet generation (HFE-7500 mechanical oil with 1.2% Bio-RAN), were used. These solutions were loaded into five separate inlets on a microfluidic chip via pressure control pumps (LineUp Flow EZ, Fluigent). The three aqueous solutions were mixed in a single channel before reaching the droplet junction, where droplets were formed by oil flow at 100 µl h−1. By varying the flow rates of the protein, RNA and buffer solutions between 5 and 54 µl h−1 while maintaining a constant flow of 21 µl h−1 for the binder (0 µl h−1 for the control), droplets of uniform size and binder concentration but varying protein and RNA concentrations were generated. These droplets were incubated for 4 min in the microfluidic chip as they moved through the incubation channel before entering a wider imaging chamber, where their flow slowed, allowing imaging. Droplets were imaged in continuous flow every 4 s using an openFrame epifluorescent microscope (Cairn Research) equipped with a ×10 air objective (Nikon CFI Plan Fluor) and a dichroic filter set (Cairn Research) to simultaneously capture two wavelengths (488 nm and 647 nm). Crosstalk calibration images were acquired by flowing single-dye droplets through the chip, with fluorescence in the other two channels used for crosstalk correction. Microscopic images of both phase-separated and homogeneous droplets were analysed using a custom Python script (Python v3.9.7). Droplets were identified through circle detection and filtered based on shape and radius to exclude erroneous detections. For each wavelength, fluorescence intensity — after illumination background subtraction — was mapped to a linear intensity–concentration fit, defined from the 1st to 99th percentile of fluorescence intensity relative to stock concentration. A convolutional neural network, trained on human-annotated data, classified droplets as either phase separated or homogeneous. Phase diagrams were generated, with each data point representing an individual droplet. Colouring reflects the local average of phase-separation classification in droplets with similar compositions, providing a consensus measurement of phase-separation probability across phase space.
Statistics and reproducibility
All experiments, including those shown in Figs. 5a,b and 6a,b, were independently repeated at least three times with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.