Research animals and procedures
A total of 65 rhesus macaques (M. mulatta) of Indian origin were utilized in this study (Extended Data Tables 1 and 2). All macaques were born and raised at the California National Primate Research Center (CNPRC). The maintenance and handling of the animals adhered to the United States Department of Agriculture (USDA) Animal Welfare Act and regulations, as well as the Guide for the Care and Use of Laboratory Animals. The animal care and use programme of The University of California, Davis, which oversees the CNPRC, is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, registered with the USDA, and maintains a Public Health Services Assurance. All procedures and experiments conducted for this study received approval from the University of California-Davis’s Institutional Animal Care and Use Committee (protocol numbers 21768, 21884, 20609, and 22373).
The primary criterion for enroling macaques in experiments involving gene therapy with AAV vectors was seronegativity for the relevant AAV capsid. To identify eligible macaques, pregnant adult females were screened for serum IgG antibodies against AAV-1 and/or AAV-8 by ELISA (see ‘Estimation of serum anti-AAV antibodies’ section) and those that were seronegative for the capsid(s) of interest were placed on hold. Dams with a history of prior live births were prioritized. The infants in groups 1–5 were vaginally delivered by dams in the outdoor colony. Those that were assigned to groups requiring AAV vector administrations at birth were removed from their mothers within 48 h of delivery and transferred to the nursery for specialized care and monitoring. Nursery-reared infants were fed a nutritionally balanced formula (Enfamil Lipil + Iron) and provided with surrogate companions, such as soft toys and blankets, to promote social and emotional development. Trained animal care staff closely monitored the infants’ health, growth and behaviour, ensuring that their physical and psychological needs were met. The infants received regular veterinary check-ups and were gradually introduced to solid foods and social housing with age-matched peers as part of their normal developmental process. The CNPRC nursery-rearing standards are designed to minimize stress and maximize the infants’ well-being, in accordance with current best practices for nonhuman primate husbandry and care in research settings. Nursery-reared infants also received high-protein chow as a supplement to formula. The diet converged over time to high-protein chow at the age of six months. All monkeys received seasonal fruits as part of the CNPRC feeding enrichment programme. Group sizes were dictated by the limited availability of AAV-8 seronegative monkeys. At least six AAV-treated and six control macaques were used in each SHIV challenge study. Efforts were made to balance groups for sex, so allocation of monkeys to each group was not random. Animal staff were not blinded to group assignments.
To identify the mothers of the infants that would be assigned to groups 7–11, we used ELISA to screen archived sera and sera collected during bi-annual physical exams for anti-AAV-8 antibodies. AAV-8 seronegative mothers-to-be were identified and their status was confirmed by a second ELISA. Monkeys that remained AAV-8 seronegative after the second ELISA were moved to indoor housing. At the time of this relocation and four weeks later, additional serum samples were collected for confirmatory AAV-8 ELISAs. If a monkey seroconverted during this quarantine screening period, it was excluded from the cohort and returned to the colony. Following three rounds of AAV screening, 16 pregnant dams remained AAV-8 seronegative and were ultimately assigned to groups A–C (Extended Data Table 2). These monkeys were then transferred to an ABSL-2 environment with specific personal protective equipment requirements to avoid transmission of wild-type AAV from the outside.
The 16 AAV-8 seronegative pregnant dams in groups A–C were scheduled to undergo caesarian section when their pregnancies reached term (approximately gestational week 24), but two dams (rhA-1 and rhA-3) delivered their babies vaginally ahead of time (Extended Data Table 2). The remaining 14 dams underwent caesarian section, which was performed by experienced veterinarians boarded by the American College of Laboratory of Animal Medicine. Initially, inhalant anaesthesia was administered, followed by surgical preparation. A laparotomy incision was made at the caudal ventral abdominal midline, and the uterus was then exteriorized. A uterotomy incision was carefully made to avoid placental sites, and the neonate was delivered. Neonatal resuscitative procedures were initiated, and the neonate was transferred to a warm workstation for monitoring. The uterotomy and the abdominal wall were closed using absorbable sutures in layers. Oxytocin was administered as needed during or after uterine closure. Following surgery, the dams were monitored in the recovery room or home cage until they maintained an upright posture. Post-surgical analgesics were administered according to standard protocols.
To deliver the recombinant bNAbs to the pregnant dams in groups B and C, the monkeys were sedated and the site over the vein where the catheter was to be placed was shaved. The area was then cleaned with gauze soaked in alcohol. During this preparation, sterility of the catheter end, which was to be inserted into the vein, was maintained. The catheter, with its stylette-bevel up, was placed on the skin. The skin was punctured with the catheter stylette, which was then introduced into the vein. While holding the stylette stable, the catheter was advanced into the vein. The stylette was then withdrawn while the catheter was held in place. An injection cap or flush syringe was attached to the catheter. The placement of the catheter was checked by flushing with a small volume of flush or fluid solution, and careful observation was made to ensure the fluid flowed into the vein without any leakage into the subcutaneous area. The catheter was then taped to the limb to secure it. For the slow infusion procedure, 30 mg kg−1 of recombinant rh-3BNC117-IgG1 or rh-3BNC117-IgG1-LS proteins was delivered through the intravenous catheter at a rate of approximately 2.0 ml min−1. The intravenous line was flushed with saline at the end of the infusion to ensure the full amount of the bNAbs was delivered.
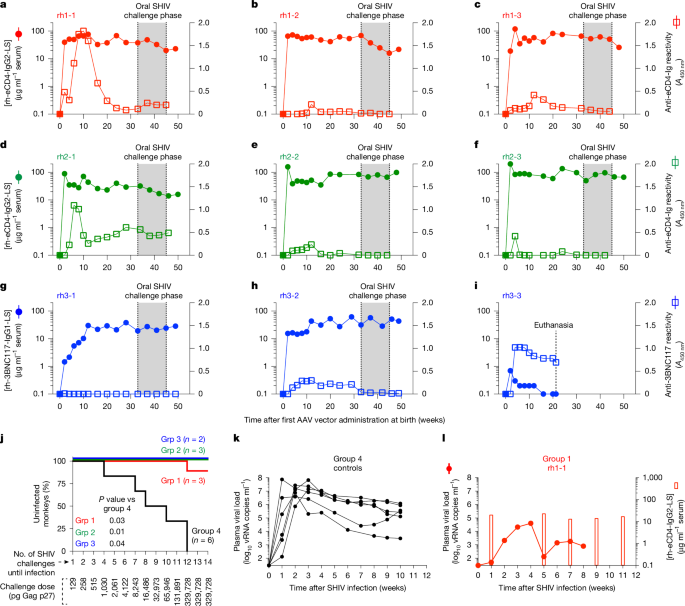
Monkeys had to weigh at least 0.5 kg to be eligible for AAV vector inoculations. The group 1 infants were injected intramuscularly with a phosphate buffered saline (PBS) solution containing weight-adjusted amounts of two AAV-1 vectors: one expressing rh-eCD4-IgG2-LS (2.0 × 1012 genome copies (GC) per kg; VCAV-05105) and the other encoding rhesus tyrosine-protein sulfotransferase-2 (TPST-2; 5.0 × 1011 GC kg−1; VCAV-05102). TPST-2 is needed during cellular synthesis of eCD4-Ig to sulfate the tyrosines in its co-receptor mimetic peptide. The sulfopeptide in the AAV-encoded rh-eCD4-IgG2-LS molecule was mim6 (GGGGGDYYDYDGGYYYDGD). Each monkey received 400 μl of this vector inoculum, which was split evenly between 2 injections into each quadriceps muscle. The group 1 infants were treated with the aforementioned AAV-1 vectors within 24 h after birth.
The group 2 infants were treated intravenously with an AAV-8-rh-eCD4-IgG2-LS (5.0 × 1012 GC kg−1; VCAV-05106) vector within 24 h of birth. Because the majority of intravenously administered AAV particles end up in the liver, where TPST-2 is constitutively expressed, no TPST-2-expressing AAV vector was delivered at this stage. The AAV-8-rh-eCD4-IgG2-LS inoculum consisted of 500 μl of PBS containing a weight-adjusted dose of the vector. At postnatal week 4, the group 2 infants were treated intramuscularly with the same doses of the same AAV-1-rh-eCD4-IgG2-LS and AAV-1-TPST-2 vectors given to the group 1 infants. This AAV-1 inoculum was formulated and administered as described above for group 1.
The group 3 infants were treated intramuscularly with 400 µl of a PBS solution containing 2.0 × 1012 GC kg−1 of an AAV-8-rh-3BNC117-IgG1-LS (VCAV-04757) vector within 24 h of birth. The AAV-8-rh-3BNC117-IgG1-LS inoculum was formulated and administered as described above for group 1. The group 3 monkey rh3-3 was euthanized at postnatal week 21 so that the remaining infants in groups 1–3 could be transitioned to paired housing.
Beginning at postnatal weeks 30–34, the monkeys in groups 1–4 (except for rh3-3) were subjected to weekly oral challenges with escalating doses of a SHIV-AD8EO stock produced in HEK293T cells. The concentration of this SHIV-AD8EO stock was 6.6 × 105 pg ml–1 of Gag p27. For the first oral challenge, each macaque was exposed to 129 pg Gag p27, which corresponded to a 5,000-fold dilution of the stock. The appropriately diluted virus inoculum was loaded into syringes without needles in a final volume of 0.5 to 1.0 ml. The challenge dose was doubled after each exposure. Beginning at the 12th exposure, the challenge inoculum consisted of 0.5 ml of the undiluted SHIV-AD8EO stock. Plasma was collected at the time of each challenge and tested for the presence of viral RNA (vRNA). Monkeys that remained aviraemic continued to be re-challenged in the next week. However, once a macaque experienced two consecutive episodes of positive viraemia, it was deemed to be infected and did not receive additional virus challenges. Plasma viral loads were then monitored for up to ten weeks post-infection.
The macaques in groups 5 and 6 were treated intramuscularly with the same dose of AAV-8-rh-3BNC117-IgG1-LS given to the group 3 infants, but the vector lot was different (VCAV-06302). The AAV-8-rh-3BNC117-IgG1-LS inoculum was formulated in PBS as described above and split into four and eight separate muscle sites for the group 5 and group 6 monkeys, respectively. Of note, the group 5 infant rh5-6 was born below the pre-specified weight criterion (≥0.5 kg) to be inoculated with an AAV vector. It took four weeks for monkey rh5-6 to gain enough weight to meet this criterion, so rh5-6 was treated with AAV-8-rh-3BNC117-IgG1-LS one month after its group 5 counterparts.
The macaques in groups 7–11 were treated intramuscularly with 2.5 × 1012 GC kg−1 of the same lot of AAV-8-rh-3BNC117-IgG1-LS (VCAV-06302) given to groups 5 and 6. The AAV-8-rh-3BNC117-IgG1-LS inoculum was formulated in PBS and split into four separate muscle injections, as described above.
When the group 5 macaques reached ~2.5 years of age, they were subjected to weekly intrarectal challenges with a peripheral blood mononuclear cell (PBMC)-derived stock of SHIV-AD8EO. The six rhesus macaques in group 12 served as controls for this experiment. The SHIV-AD8EO stock used for this experiment was amplified in concanavalin A-activated rhesus PBMCs for 7 days. The Gag p27 concentration of the resulting stock was 96 ng ml–1 and each monkey in groups 5 and 12 was challenged intrarectally with 3.8 ng of Gag p27 of this stock. The challenge inoculum for each macaque consisted of 1.0 ml of PBS containing 0.5% of fetal bovine serum and 1:25 dilution of the aforementioned SHIV-AD8EO stock. This volume was loaded into 3-ml syringes and applied intrarectally (without needle) to each macaque. Plasma was collected at the time of each challenge and tested for the presence of vRNA. Monkeys that remained aviraemic continued to be re-challenged in the next week. However, once a macaque experienced two consecutive episodes of positive viraemia or a single episode with a viral load greater than 1,000 vRNA copies ml–1 of plasma, it was deemed to be infected and did not receive additional virus challenges. Plasma viral loads were then monitored for 10 weeks after infection.
SHIV RNA viral load measurements
Three hundred and fifty-microliter EDTA plasma samples were collected from the infant macaques in groups 1–4 during the oral SHIV-AD8EO challenge phase. These samples were diluted 1:1 with TRIS buffer and then RNA was extracted and analysed as previously described51. The threshold of detection on an input volume of 0.35 ml of plasma was 29 vRNA copies ml–1.
Plasma viral loads from rhesus macaques in groups 5 and 12 were measured using 0.5 ml of EDTA-anticoagulated plasma based on a modification of a previously published method51. The threshold of detection on an input volume of 0.5 ml of plasma was 15 vRNA copies ml–1.
Production of monoclonal antibodies for in vivo studies
The recombinant rh-3BNC117-IgG1 and rh-3BNC117-IgG1-LS proteins used in groups B and C were expressed by Chinese hamster ovary (CHO)-K1SP cell lines stably expressing each molecule. Both cell lines were developed and authenticated by Genscript, but they were not tested for Mycoplasma contamination. In sum, linearized plasmids encoding each molecule were transfected into CHO-K1SP cells, followed by cell pool screening and selection. The selected pools were then subjected to single clone screening using the limiting dilution method, followed by single clone batching in six-well plates. After the first batch of single clones was identified, the top six were selected for fed-batch. Based on their growth performance and productivity, the top three clones were selected for final delivery. Both molecules were purified from supernatant by affinity chromatography, followed by cation exchange and ultrafiltration–diafiltration. Both molecules were formulated in citrate buffer (20 mM sodium citrate, 150 mM sodium chloride, pH 6.0, 0.02% Tween-80) at a concentration of ~10.5 mg ml–1. The endotoxin level was less than 1.0 endotoxin units per mg of protein, as determined by the limulus amoebocyte lysate (LAL)/tachypleus amebocyte lysate (TAL) assay. The rh-3BNC117-IgG1-LS molecule (lot U086PGG21009/202201) was produced and purified by Genscript, and the rh-3BNC117-IgG1 protein (RRID: AB_2895627, lot JH21-08) was produced and purified by MassBiologics.
AAV vectors
Three single-stranded AAV transfer plasmids were used to produce the recombinant AAV vectors described in this study. The first one encoded rh-3BNC117-IgG1-LS and was used to prepare the AAV-8 vector used in group 3 and groups 5–11. Transgene expression was under the control of the cytomegalovirus (CMV) promoter. A simian virus 40 (SV40) intron was placed between the promoter and the rh-3BNC117-IgG1-LS transgene. To reduce the immunogenicity of this vector, the promoter and transgene sequences lacked CpG motifs that can serve as ligands for TLR952. The rh-3BNC117-IgG1-LS transgene was bicistronic; that is, both heavy and light chains of IgG1 were expressed from one open reading frame using an F2A ‘self-processing’ peptide from foot-and-mouth disease virus. The cleavage sequence RKRR for the cellular protease furin is added for removal of amino acids that were left on the heavy chain C-terminus following F2A self-processing. The peptide linker SGSG is added for improved furin enzyme-mediated cleavage. Additionally, the 3′ untranslated region contains multiple binding sites for conserved endogenous microRNAs (miRNAs) that are specifically expressed in professional antigen-presenting cells (pAPCs). These miRNA binding sites were included to render the monoclonal antibody transcripts sensitive to translational inhibition by the miRNAs expressed in pAPCs. The second AAV expression cassette encoded a rh-eCD4-IgG2-LS molecule and was used to prepare the AAV-1 and AAV-8 vectors used in groups 1 and 2. Additional relevant features about the rh-eCD4-IgG2-LS protein include an I39N substitution in the CD4 domains intended to increase HIV neutralization potency and the use of mim6 (GGGGGDYYDYDGGYYYDGD) as the carboxyl-terminus co-receptor mimetic peptide. Transgene expression was under the control of the chicken β-actin promoter with a CMV enhancer and an SV40 intron. Downstream from the rh-eCD4-IgG2-LS transgene was the woodchuck hepatitis virus posttranscriptional regulatory element, which was intended to increase transgene expression. The third AAV expression cassette encoded rh-TPST-2 and was used to prepare the AAV-1-rh-TPST-2 construct that was co-delivered with the AAV-1-rh-eCD4-IgG2-LS vector during the intramuscular inoculations of the group 1 and group 2 monkeys. The promoter structure of this vector consisted of the CMV promoter and CMV enhancer, followed by the SV40 intron. All AAV expression cassettes used in this study contained the SV40 poly-adenylation signal and were flanked by AAV-2 inverted terminal repeats. All recombinant AAV vectors used in this study were produced at the Horae Gene Therapy Center and Vector Core at the UMass Chan Medical School as described previously53. In brief, HEK293T cells (American Type Culture Collection, authenticated and tested negative for Mycoplasma contamination) were co-transfected with each transfer plasmid, a plasmid co-expressing the AAV-2 Rep protein with the AAV-1 or AAV-8 capsid, and a helper plasmid encoding adenovirus genes. After collecting lysates of transfected cells, AAV was purified through three sequential CsCl centrifugation steps. The vector GC number per ml was determined by quantitative PCR. The purity of the AAV preparations was verified by silver-stained SDS–PAGE.
RNA-seq analysis
Following collection of peripheral blood (~0.5 ml per time point) in BD PAXgene tubes, the tubes were gently inverted 8–10 times and stored vertically at room temperature for a minimum of 2 h. The tubes were then transferred to a −80 °C freezer where they were stored until the time of the RNA extraction. Total RNA was extracted using a Paxgene Blood miRNA kit (Qiagen 763134), following the manufacturer’s instructions. The purified RNA was eluted in buffer BR5 (supplied in the kit), incubated at 65 °C for 5 min for RNA denaturation, and stored immediately at −80 °C. RNA samples were shipped on dry ice to Novogene for RNA quality control, library preparation and RNA sequencing. cDNA libraries were prepared at Novogene from total RNA using the NEBNext Ultra II RNA Library Prep Kit for Illumina (NEB E7770L). Sequencing was performed on a Novaseq 6000 instrument (Illumina).
After initial quality control, the sequence data were quantified using Kallisto54, to obtain transcript level abundances using Mmul_10 (Ensembl) as reference. Following quantification, DEGs and differentially expressed transcripts (DETs) were identified using Sleuth55. The DETs were identified in samples collected on day 3 and week 4 vs. baseline (day 0) post-AAV vector administration in group 5 and group 6, respectively. We performed a similar analysis in infants in group 7 and in those in groups 8 and 9. Significant DETs were defined using a q-value (Benjamini–Hochberg adjusted P value) threshold of <0.05. Over-representation analysis was performed using the Clusterprofiler56,57 tool to identify enrichment of the genes in specific pathways described in Kyoto Encyclopedia of Genes and Genomes (KEGG)58 and Reactome59 databases. The gene counts for groups 5–9 in Extended Data Figs. 6 and 7 ranged from 20,515 to 28,321.
Estimating monoclonal antibody concentrations in serum
Concentrations of rh-3BNC117-IgG1-LS in macaque serum were quantified by ELISA as follows: 384-well high binding polystyrene plates (Corning 3700) were coated with 4.0 μg ml–1 of the anti-idiotype antibody 1F1 (Protein Production Facility, The Duke Human Vaccine Institute, Duke University, NC) diluted in Dulbecco’s PBS without Ca2+ or Mg2+ (DPBS) for 1.5 h at 37 °C. The plate was washed twice with 10× diluted commercial 0.5% Tween-20 solution (BioWorld 40120769-3) and blocked with 5.0% (w/v) bovine serum albumin (BSA) in DPBS (blocking buffer) for 1 h at 37 °C. The blocking buffer was aspirated off the plate and serum samples, serially diluted in the blocking buffer, were loaded onto the plate. Each assay contained serial dilutions of recombinant rh-3BNC117-IgG1-LS to generate a standard curve. These serial dilutions were performed with blocking buffer and the first well of the series was spiked with macaque serum to a final dilution of 1:2,000. The samples and the standard curve dilutions were incubated in the plate for 1.5 h. The plate was then washed five times. Next, a (Fab)2 fragment conjugated to horseradish peroxidase (HRP) specific for the human Fcγ domain (Jackson Immuno 109-036-008) was diluted 1:5,000 in blocking buffer. This solution was added to the plate and incubated at 37 °C for 1 h. The plate was washed 10 times and developed using 1-step Ultra TMB-ELISA Substrate solution (Thermo Fisher 34028). The reaction was stopped using Liquid Stop Solution for TMB (Surmodics LTSP-1000-01). Absorbance was measured at a wavelength of 450 nm using a BioTek Synergy LX spectrophotometer (Agilent Technologies). To obtain an ELISA standard curve, the recorded absorbance values were plotted on the y axis, while the concentrations of the standard protein, namely rh-3BNC117-IgG1-LS, were plotted on the x axis. Data analysis and curve fitting were performed with GraphPad Prism software, employing a four-parameter logistic or sigmoidal curve model. Subsequently, this model was used for the estimation of unknown concentrations of the analyte within the serum samples via interpolation. Interpolated values derived from the linear region typically in mid to low concentration ranges of the standard curve were utilized for further analyses. The same methodology was used to determine serum concentrations of rh-eCD4-IgG2-LS, except that plates were coated with 4.0 μg ml–1 of a mouse anti-CD4 monoclonal antibody (clone MEM-241; Millipore Sigma SAB4700059) and serial dilutions of recombinant rh-eCD4-IgG2-LS were used to generate the standard curve of the assay.
Estimation of ADA responses in serum
Serum reactivity to rh-3BNC117-IgG1-LS was monitored by ELISA using 384-well high binding polystyrene plates (Corning 3700). These plates were coated with a DPBS solution containing 2.0 μg ml–1 of purified recombinant rh-3BNC117-IgG1-LS, which uses a κ light chain. Each plate was washed twice with 10× diluted commercial 0.5% Tween-20 solution and blocked with 5.0% (w/v) BSA in DPBS for 1 h at 37 °C. The blocking buffer was aspirated off the plate and serum samples, diluted 1:200 in the blocking buffer, were loaded onto the plate. Samples were incubated in the plate for 1.5 h. The plate was then washed five times. Next, a goat polyclonal antibody preparation specific for human IgG λ light chain and conjugated to HRP (Southern Biotech 2070-05) was diluted 1:5,000 in blocking buffer. This solution was added to the plate and incubated at 37 °C for 1 h. The plate was washed 10 times and developed using 1-step Ultra TMB-ELISA Substrate solution (Thermo Fisher 34028). The reaction was stopped using Liquid Stop Solution for TMB (Surmodics LTSP-1000-01). The same 384-well ELISA plate was used to screen all the group 5 and group 6 samples for anti-rh-3BNC117-IgG1-LS antibodies. A separate plate was used for groups 7–11.
The same methodology was used to measure anti-rh-eCD4-IgG2-LS antibody responses. However, because rh-eCD4-IgG2-LS does not have light chains, reactivity was assayed with detector antibodies against both κ (Southern Biotech 2060-05) and λ light chains, both diluted 1:5,000 in blocking buffer. All serum samples from monkeys in groups 1 and 2 were tested at a fixed serum dilution of 1:20. Reactivity against each AAV-delivered molecule was plotted as background- and baseline-subtracted absorbance values measured in each assay.
SHIV neutralization assay
TZM-bl neutralization assays were performed as described60. In brief, rhesus serum was serially diluted, mixed with SHIV-AD8EO Env-pseudotyped SG3.1 pseudovirus, and incubated at 37 °C, 5% CO2 for 1 h. TZM-bl cells (NIH AIDS Reagent Program; not authenticated) were then added to each well and the plate was incubated for an additional 48 h. DEAE dextran was added to the cell containing media before its addition to the plate, such that the final concentration in each well was 15 μg ml–1. Plates were developed using Britelite reagent (PerkinElmer 6066761) and luciferase activity, measured as total luminescence, was quantified using a Synergy LX plate reader (Agilent Technologies). Raw luminescence values were converted to ‘per cent neutralization’ using the formula [1 − (sample luminescence − background luminescence)/(virus control − background luminescence)] × 100 and plotted in GraphPad Prism software. ID50 titres were calculated by fitting each neutralization curve with a 4-parameter logistic non-linear regression, and then interpolating 50% neutralization onto each curve. The TZM-bl cell line tested negative for Mycoplasma contamination.
Estimation of serum anti-AAV antibodies
ELISA plates (384-well high binding polystyrene plates; Corning 3700) were coated with rAAV-8 viral particles by adding 30 µl of AAV-8, diluted to 1.55 × 1010 GC ml–1 in DPBS without Ca2+ or Mg2+, to each well. Coating was allowed to proceed overnight at 4 °C. Plates were then washed twice with a commercial wash buffer (BioWorld 40120769-3) and blocked by adding 90 µl of 5.0% Blotto (Rockland Immunochemicals B5010500) in DPBS followed by incubation at 37 °C for 1 h. The plate was aspirated and serum samples, diluted by a factor of 200 in the same blocking buffer, were loaded onto the plate. The plate was incubated at 37 °C for 1 h and then washed 5 times. A Fab fragment conjugated to HRP, specific for human Fcγ (Jackson Immuno 109-036-008) was added to each well and the plate was incubated at 37 °C for 1 h. The plate was then washed 10 times and developed using 1-step Ultra TMB-ELISA Substrate solution (Thermo Fisher 34028) and Liquid Stop Solution for TMB (Surmodics LTSP-1000-01). All samples in the study were measured on the same ELISA plate, so that valid relative comparisons could be made using raw absorbance values.
Statistics
Kaplan–Meier survival analysis was used to determine whether the rate of SHIV-AD8EO acquisition differed between AAV-treated and control macaques following oral or intrarectal challenges with SHIV-AD8EO. The P values for these comparisons were calculated using the log-rank (Mantel-Cox) test. Differences between serum concentrations of rh-3BNC117-IgG1-LS, anti-rh-3BNC117-IgG1-LS antibody responses (that is, ADAs) and other immune parameters were assessed using the Mann–Whitney U-test.
For comparisons of ADAs between groups, we determined the cumulative levels of these responses for each monkey. Specifically, we calculated the AUC of absorbance values measured during the 20 weeks of follow-up after the AAV vector administration. To avoid confounders related to inter-assay variability in ADA values, only absorbance values measured in the same 384-well ELISA plate were used to calculate AUC values. For these assays, the developing reagents were added to the plates within seconds using an automated 96-channel pipette in order to minimize time differences in well development.
The Spearman rank correlation method was used to search for associations between the age of monkeys at the time of AAV-bNAb vector administration and bNAb serum concentrations and the cumulative levels of anti-bNAb antibody responses measured over time. The incidence of macaques that developed persistent bNAb expression following AAV-bNAb therapy was compared between experimental or age groups using Fisher’s exact test. A significance threshold of 0.05 was used for all statistical tests. All P values reported here are two-tailed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.