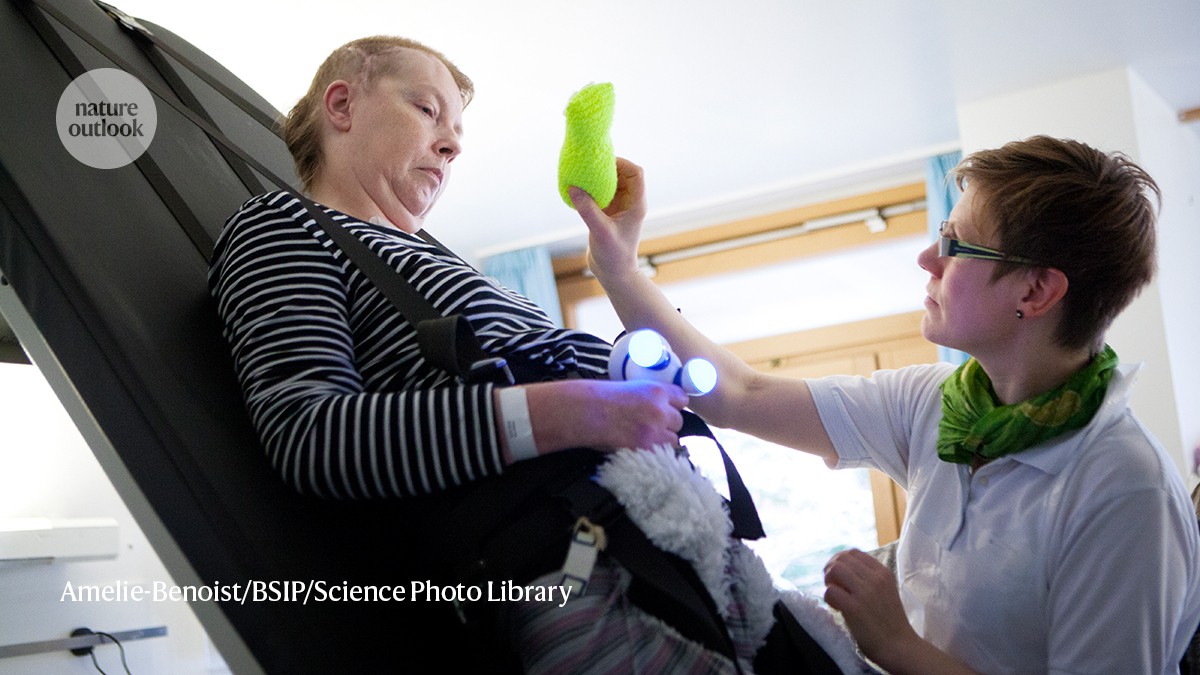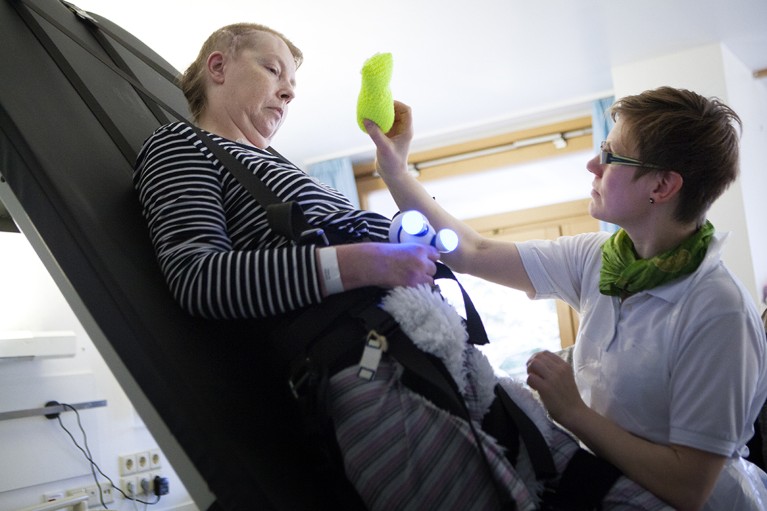
A physiotherapist assists a woman who has experienced a traumatic brain injury with rehabilitation exercises. Credit: Amelie-Benoist/BSIP/Science Photo Library
It’s the middle of the night. A young mother makes her way into her crying infant’s bedroom. Her toddler is fast asleep in a bed opposite the newborn’s crib. After picking up the baby, the mother trips over a toy and falls backwards. She doesn’t drop the baby, but hits the back of her head on the toddler’s bed frame. The woman never loses consciousness, but for the rest of the night and the following day, she has a splitting headache, vision problems and dizziness. Her physician orders a computed tomography (CT) scan and magnetic resonance imaging (MRI), which show there is nothing wrong with her brain. According to imaging, she should be fine, so the physician sends her home despite her ongoing symptoms.
Typically, the woman would have no further recourse, but she happened to learn about a clinical study close to her home at Bellevue Hospital in New York City. Uzma Samadani, a neurosurgeon and brain-injury researcher now at the University of Minnesota in Minneapolis, was testing a device that analyses eye movement to spot the signs of traumatic brain injury (TBI). When her test came back unmistakably abnormal, the woman started crying, Samadani recalls. “She said, ‘I feel so vindicated because nobody believed me.’”
Part of Nature Outlook: Medical diagnostics
Samadani thinks that the mother’s story exemplifies the problem that people run into after experiencing a mild TBI, when consciousness is lost either transiently or not at all. Although mild TBIs, which include concussions, account for 70–90% of TBI cases, there is no generally accepted standard for diagnosing one.
What do exist are clinical assessment tools, such as the Glasgow Coma Scale, which scores a person’s verbal and motor responses, as well as their ability to open their eyes. But such tests can be subjective, and become less useful when people are inhibited by alcohol, intubation or a co-occurring event such as a stroke, for example. In those cases, clinicians often turn to imaging techniques. But these technologies, Samadani says, are not sensitive enough to identify the microscopic damage that is characteristic of mild TBIs.
“If you are brought to an emergency room with a traumatic brain injury and your CT scan is negative, you are almost always told ‘you’re fine, go home’,” says David Okonkwo, a neurosurgeon and brain-trauma researcher at the University of Pittsburgh Medical Center in Pennsylvania. “But we now understand that a significant percentage of those patients are not fine, and in fact continue to have issues for months.” The young mother that Samadani saw took six months to fully recover from her injury.
A solution to this diagnostic problem would be easily measured biomarkers that indicate whether a person has a mild TBI. Such techniques could help to ensure that people with a mild TBI are identified for follow-up care throughout their potentially long recovery. The same tests could also help to stop athletes returning to play too soon, or help military medical personnel to make critical decisions in settings in which a CT scan isn’t feasible.
After decades of work, researchers are now using a variety of techniques to search the blood, eyes and saliva for diagnostic biomarkers of mild TBIs. No single method has been approved as a standalone diagnostic, and the biomarkers identified so far need more data to support them before their role in clinical care can be fully determined, says David Brody, a neurologist at the Uniformed Services University in Bethesda, Maryland, and editor-in-chief of the Journal of Neurotrauma. But researchers are encouraged by blood tests and devices to aid in mild-TBI diagnosis that have received regulatory clearance in the past six years. “I am as excited and optimistic about what we can do for patients with TBI in the coming years as I have ever been in my entire career,” Okonkwo says.
Clues in blood
As a graduate student in the 1990s, Okonkwo studied trauma-related changes in the brain using antibodies that bound to proteins such as UCH-L1, found in central nervous system (CNS) neurons, and GFAP, found in CNS astrocytes. It didn’t cross his mind that these brain-specific proteins could also show up in blood. “Twenty five years ago, that was considered magical thinking — that you could ever measure a problem with someone’s brain through a blood test,” he says.

Kevin Wang studies biomarkers for traumatic brain injuries at Morehouse School of Medicine in Atlanta, Georgia. Credit: Troy Cai
But a group of scientists at the University of Florida in Gainesville didn’t consider it to be so fanciful. In the mid-2000s, the researchers identified potential biomarkers by analysing rat brain tissue and blood after the animals sustained controlled head injuries. Among these researchers was Kevin Wang, now at Morehouse School of Medicine in Atlanta, Georgia. Wang found that in blood samples from animals and people who had sustained a TBI, levels of UCH-L1 and GFAP were consistently higher than normal1. Encouraged by these results, Wang and his colleagues formed the company Banyan Biomarkers in Alachua, Florida, in 2002.
In a study of 1,959 frozen human blood samples, funded in part by Banyan, researchers reported that high levels of UCH-L1 and GFAP in the blood predicted CT-detectable brain damage in 97% of people with a mild TBI2. On the basis of these results, in 2018, the US Food and Drug Administration (FDA) granted clearance for the test to be used to see whether a person needs a CT scan. Although that’s a narrow indication for use, Wang says it was a big step. This is because in at least 90% of people with a mild TBI who receive a CT scan, the scan shows no apparent damage. The upshot is that a lot of people, including children, are being subjected to unnecessary radiation. “We can reduce some of that,” Wang says.
AI’s keen diagnostic eye
Since 2018, Banyan has licensed the test to Abbott Laboratories in Chicago, Illinois, and to bioMérieux in Marcy-l’Étiole, France. A consortium of TBI researchers called Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) worked closely with Abbott to validate the company’s own version of the test on thousands of frozen plasma samples, leading to FDA clearance in 2021. Meanwhile, bioMérieux gained clearance from the European Commission in 2023 and the FDA in 2024. In April 2024, the FDA approved a portable version of Abbott’s test that measures GFAP and UCH-L1 levels from a small sample of fresh whole blood in 15 minutes.
Okonkwo, who is one of TRACK-TBI’s principal investigators, says that the consortium is working with Abbott to uncover further uses for the test — including diagnosing mild TBIs that don’t show up on CT imaging. TRACK-TBI has also started a paediatric study to expand the age range for the current version of the test, which is approved only for adults.
Jessica Gill, a neurobiologist at Johns Hopkins University in Baltimore, Maryland, says that the test is merely a first step towards the development of blood-based mild-TBI diagnostics. Gill’s team has started a project in collaboration with US life-sciences firm Danaher, based in Washington DC, using frozen blood samples from people with mild a TBI to develop tests that can diagnose an injury, provide information about its severity and track recovery in health-care settings. And there are dozens of other protein biomarkers in the discovery phase, according to Brody.
Gill’s group has fished out several undisclosed protein candidates that it will test in combination with established biomarkers, such as GFAP, in a prospective study that will enrol people from emergency departments in the Johns Hopkins network.
Significance of spit
Proteins aren’t the only molecules released from the brain after injury. There is growing interest in measuring nucleic acids called microRNAs, which regulate protein production by binding to messenger RNAs. All cells release microRNAs, and when a tissue is injured, the profile of the released microRNAs changes.
Steven Hicks, a paediatrician and neuroscientist at Pennsylvania State University’s Neuroscience Institute in Hershey, is studying microRNAs in children. Soon after starting this research in 2012, he realized it’s much easier to get children to spit than to give blood. That suited him just fine, he says, because microRNAs in saliva are both more abundant than in blood and, crucially, more likely to come from the brain, owing to the contact of cranial nerves with the throat and mouth.
Hicks concluded that a saliva-based test for mild TBIs might be especially useful for children and young athletes. When he began testing the idea, he tended to get funny looks from colleagues, but one study involving mixed martial arts fighters bolstered his persistence3. Hick’s team collected saliva and blood from 50 fighters before and after matches and then for a week after. The study identified changes to microRNAs that correlated with the number of blows each fighter took to the head. It took just an hour for microRNA levels to shift in saliva samples; by contrast, it took days to see shifts in blood. This convinced Hicks that saliva was the body fluid of choice to measure microRNAs shortly after a sports-related head injury.

Neurosurgeon Uzma Samandi is the founder of Oculogica in New Richmond, Wisconsin, which developed the eye-tracking device called EyeBOX that assesses the severity of traumatic brain injuries.Credit: Uzma Samandi
Over the past eight years, Hicks’ team has narrowed down thousands of microRNAs to just a handful associated with mild TBIs4,5. Hicks also consults for Quadrant Biosciences, a company in Syracuse, New York, that is developing a saliva-based microRNA test for concussion on the basis of his and other researchers’ work.
Gill says that microRNAs hold particular promise as a diagnostic tool in low-resource settings because they remain stable for weeks at a wide range of temperatures. By comparison, she says, “proteins in the blood will change within minutes”. Although her team is not currently developing any RNA-based tests, it is storing RNA sequence data from its samples for a possible study later.
Window into the brain
For centuries, head-injury assessment has included asking people to track an object or finger with their eyes. Loss of control over eye movement is a common sign of brain injury, but deciding whether eye movements are unusual is subject to interpretation. Samadani is one of several researchers to have designed ways to quantify these tests and turn them into objective diagnostic tools.
In 2013, Samadani founded Oculogica in New Richmond, Wisconsin, to make an eye-tracking device called EyeBOX. This product was designed to measure progress objectively in people with severe TBIs during clinical trials. Existing eye-movement tracking devices either needed a baseline measurement taken before injury, which is not a realistic expectation for people with a TBI, or involved a calibration step that required people to be able to follow instructions. Samadani wanted an objective, standalone test that didn’t rely on a person’s cognitive abilities.
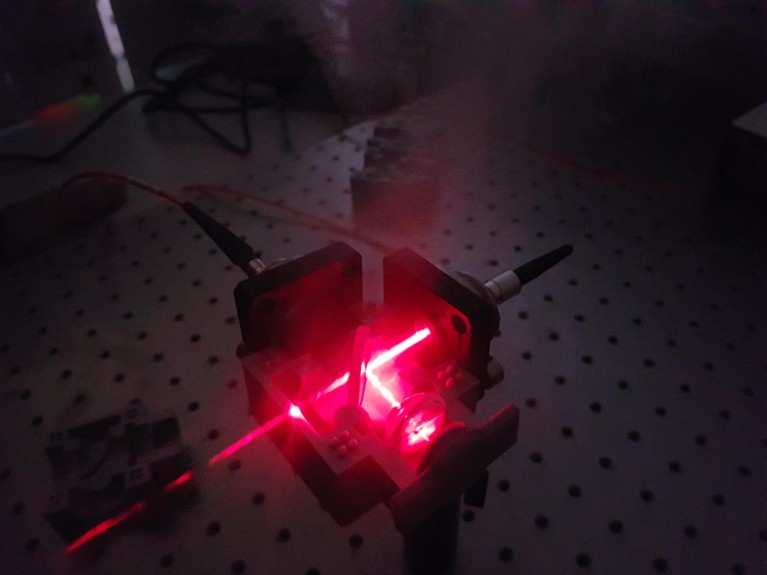
A laser device developed by physicist and chemical engineer Pola Goldberg Oppenheimer and her team at the University of Birmingham, UK, could detect whether a person has had a mild traumatic brain injury.Credit: Pola Goldberg Oppenheimer
EyeBOX records the trajectories of the right and left eye over time as a person watches a video. An associated algorithm compares the trajectories to determine how well the eyes move in unison. The content of the video is not important, Samadani says. During the initial development of the system, it was mainly music videos by the singer Shakira and Disney films. But now, she says, “we use a huge variety of content”.
In 2018, the FDA cleared EyeBOX to help diagnose concussion after a study of 282 people with head injuries showed that the device identified mild TBIs with 80% accuracy6. A more compact version of the device received FDA clearance in 2022. Both devices are approved for ages 5–67 years and within a week of head injury.
Although eye-movement tests might provide a metaphorical window into the brain, Pola Goldberg Oppenheimer, a physicist and chemical engineer at the University of Birmingham, UK, thinks that they can also allow direct detection of biomarkers in neuronal tissue. “We know that the optic nerve is immersed in cerebrospinal fluid and connected to the central nervous system,” Goldberg Oppenheimer says. All that’s needed is a way to peek inside.
Goldberg Oppenheimer’s team has developed a device called EyeD, which shines an eye-safe laser on the neuronal tissue at the back of the eye and analyses the light spectra reflected to detect proteins and fats that could act as useful biomarkers. The team developed an algorithm to interpret the spectra and generate a score to indicate the likelihood of a mild TBI. In a study published last year7, the device and algorithm distinguished spectra generated from the eyes of pigs who died natural deaths from those that were killed after an electric stun-gun shock to the brain.
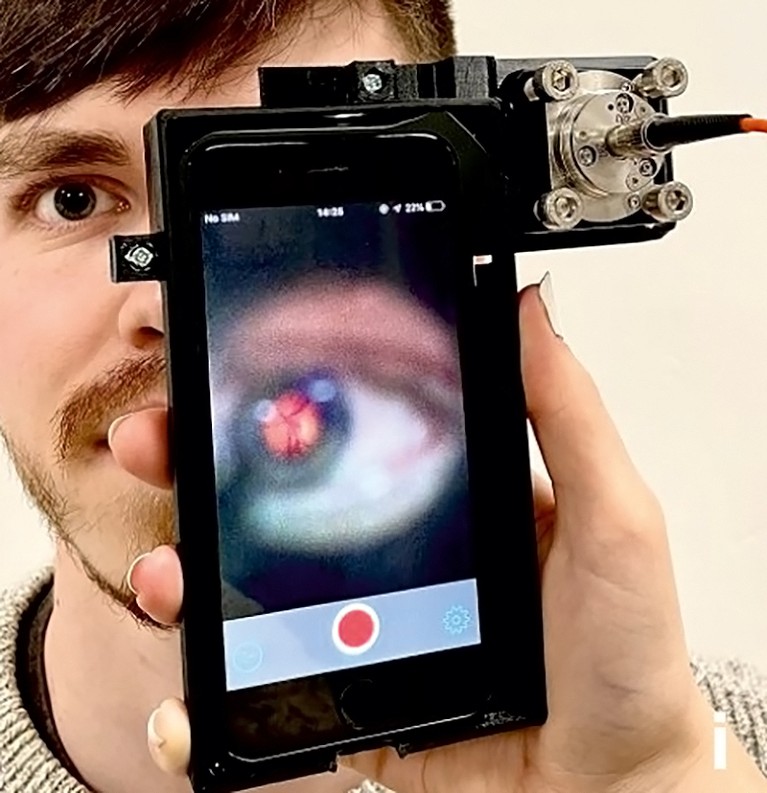
EyeD is a handheld device that shines a laser at the back of the eye to detect proteins and fats that could act as useful biomarkers of a traumatic brain injury.Credit: Ref. 7
Goldberg Oppenheimer is hoping to start a study of the EyeD device in people in 2025. The study will allow the researchers to narrow in on the most informative eye-tissue biomarkers, which Goldberg Oppenheimer expects will be distinct from those found in blood.
Each diagnostic technique stands to offer the most at different time points after injury and after different types of injury. For example, Brody says, mild TBIs caused by repeated blows over long periods of time — such as those sustained by professional athletes, soldiers and victims of domestic violence — might need a unique set of diagnostic tools.
Gill oversees biomarker research for the Long-Term Impact of Military-Relevant Brain Injury Consortium, which is collecting clinical data, blood and saliva from more than 3,000 veterans, including those who have been repeatedly exposed to explosive blasts. Study participants are undergoing MRI as well as cognitive and neuropsychiatric assessments, while researchers search their blood for protein biomarkers, including GFAP and UCH-L1. The goal, Gill says, is to identify diagnostic tests and biological signposts for later problems such as cognitive decline or psychological disorders
This work will also translate into emergency health-care settings, to help people with a mild TBI whose brains seem normal on a CT scan, like the young mother in Samadani’s study. The eventual dream, says Gill, is that clinicians will be able to warn people about possible outcomes. An emergency-room physician might be able to say, for instance, “your CT is negative, but, oh, it looks like these three biomarkers are up so you’re at high risk to develop depression”. Improved diagnostics, she adds, could help to reduce the stigma around the neuropsychiatric symptoms of a mild TBI — and guide people with the condition to think of their brain health as something that they can manage rather than just accept.