Animals
All experimental procedures were approved and carried out in accordance with the regulations of the Max Planck Florida Institute for Neuroscience Animal Care and Use Committee as per the guidelines by the US National Institutes of Health. C57/B6 mice were used. The mice were kept in 12-h lightâdark cycle at 18â21â°C with 40â50% humidity. We also used Camk2aT286A mice to test the requirement of CaMKII in BTSP experiments30.
Plasmid constructs
We fused two monomeric dimVenus (Venus(A206K,Y145W)) and mouse eGFP (eGFP(A206K)) to rat CaMKIIα subunit (2dV-Camuiα) (Addgene, 220366)8,31. T286A (Addgene, 220367) and T305D/T306D (Addgene, 220368) 2dV-Camuiα mutants were constructed by restriction digestion and ligation. To do the ER calcium imaging experiments, we used the ER-GCaMP6-210 plasmid (Addgene, 86919).
Organotypic hippocampal slice cultures and transfection
Organotypic hippocampal slices were prepared from wild-type or transgenic P4âP8 C57/B6 mouse pups of both sexes as previously described32. In brief, the animal was anaesthetized with isoflurane, after which it was quickly decapitated and the brain removed. The hippocampi were dissected and cut into 350-µm thick coronal hippocampal slices using a McIlwain tissue chopper (Ted Pella) and plated on hydrophilic PTFE membranes (Millicell, Millipore) fed by culture medium containing MEM medium (Life Technologies), 20% horse serum, 1âmM l-glutamine, 1âmM CaCl2, 2âmM MgSO4, 12.9âmM d-glucose, 5.2âmM NaHCO3, 30âmM HEPES, 0.075% ascorbic acid and 1âµgâmlâ1 insulin. The slices were incubated at 37â°C in 5% CO2. After 7â12âdays in culture, CA1 pyramidal neurons were transfected using biolistic gene transfer with 1.0âµm gold beads (8â12âmg) coated with 2dV-Camuiα (50âμg)33. For CaMKII experiments, owing to the size of the CaMKII sensor, plasmid transfection using biolistic gene gun was the most effective. Transfection was done days in vitro dayâ7â10, and experiments were performed 2â7âdays after transfection. The age of neurons would correspond to acute slices from juvenile animals34.
Acute slice preparation
Male C57/B6 mice (P25âP35 or P45âP60) were sedated by isoflurane inhalation and perfused intracardially with a chilled choline chloride solution. The brain was removed and placed in the same choline chloride solution composed of 124âmM choline chloride, 2.5âmM KCl, 26âmM NaHCO3, 4âmM MgCl2, 1.2âmM NaH2PO4, 10âmM glucose and 0.5âmM CaCl2, pHâ7.4 equilibrated with 95% O2 and 5% CO2. Coronal hippocampal slices (300âμm) from both hemispheres were cut using a vibratome (V1200, Leica) and maintained in a submerged chamber in artificial cerebrospinal fluid (ACSF; 127âmM NaCl, 2.5âmM KCl, 4âmM CaCl2, 25âmM NaHCO3, 1.25âmM NaH2PO4 and 25âmM glucose) at 32â°C for 1âh and then at room temperature in oxygenated ACSF.
Two-photon glutamate uncaging
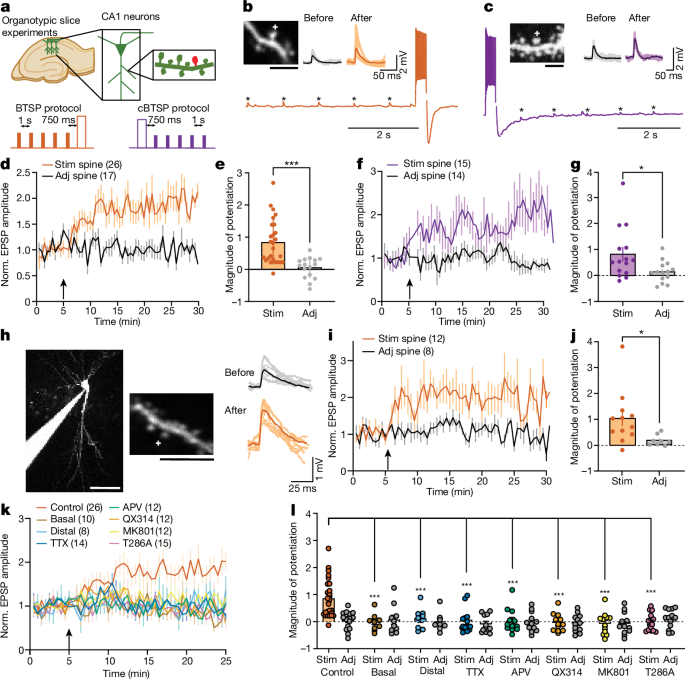
Two-photon glutamate uncaging was performed during BTSP and structural LTP experiments in organotypic hippocampal cultures and in acute hippocampal slices as previously described35,36. Experiments were performed in a small recirculating volume (about 8âml) of continuously oxygenated ACSF containing 4âmM 4-methoxy-7-nitroindolinyl-caged-l-glutamate (MNI-caged glutamate). A Ti:Sapphire laser was tuned at a wavelength of 720ânm to uncage MNI-caged glutamate in a small region about 0.5âμm from the spine. For structural plasticity experiments, 30 uncaging pulses of 0.5âHz train were given. The power of the laser was set to 2.7âmW measured at the objective. These structural plasticity experiments were performed in Mg2+-free ACSF (127âmM NaCl, 2.5âmM KCl, 4âmM CaCl2, 25âmM NaHCO3, 1.25âmM NaH2PO4 and 25âmM glucose) containing 1âμM TTX and 4âmM MNI-caged l-glutamate aerated with 95% O2 and 5% CO2. The BTSP experiments were performed in 2âmM Ca2+ and 1âmM Mg2+. Experiments were performed at room temperature (24â26â°C).
Electrophysiology
Whole-cell patch-clamp electrophysiology experiments were combined with glutamate uncaging to induce BTSP at individual dendritic spines36. The cells were first visualized in a bright field, or for the labelled cells, epifluorescence microscopy. The patch pipette (with a tip resistance of 2â5âMΩ) included the K+-based internal solution containing 145âmM K-gluconate, 14âmM phosphocreatine, 4âmM NaCl, 0.3âmM NaGTP, 4âmM MgATP, 3âmM l-ascorbic acid, 50â100âµM Alexa-594 and 10âmM HEPES (pHâ7.4, 294âmOsm). In BTSP experiments, the EPSPs were measured under the current-clamp mode by a patch-clamp amplifier (MC-700B, Molecular Devices) and digitizer (National Instruments). After 2â5âmin of dye loading, fluorescence from Alexa-594 was used to find dendritic spines in 2pFLIM. Uncaging-evoked EPSPs were induced on 1â2 spines on a dendrite by MNI-glutamate uncaging, about 0.5âµm away from the tip of the spine. The uncaging-evoked EPSP amplitude was 0.4â2âmV. Some BTSP experiments were performed in voltage-clamp configuration, for which the cells were held at â70âmV. The baseline glutamate uncaging-evoked EPSC amplitude was between 5 and 20âpA. These voltage-clamp experiments were performed with Cs+-based internal solution containing 130âmM Cs-methanosulfonate, 6âmM KCl, 10âmM HEPES, 4âmM NaCl, 0.3âmM NaGTP, 4âmM MgATP and 14âmM Tris-phosphocreatine (BTSP voltage-clamp protocol). Experiments were performed at room temperature (24â26â°C). In the CaMKII imaging experiments, similar to the above experiments, Alexa-594 dye (Thermo Scientific, 100âµM) was loaded as a structural marker. For experiments using APV, TTX, nifedipine, xestosponginâC and MK-801, slices were incubated with the drugs for more than 30âmin before BTSP experiments and were applied throughout the recording. Control experiments were done each day before the addition of the drug. For DMSO control experiments, a different ACSF solution was prepared each day. In experiments with thapsigargin and U73122, slices were incubated with the drugs for at least 60âmin before the experiments. For QX314 experiments, we started the recordings 3â5âmin after establishing the whole-cell patch clamp, and only the cells in which the BTSP protocol did not elicit any spiking during the current injection were considered for further analyses. All drugs were purchased from Tocris Biosciences unless specified otherwise. EPSPs were measured before and after the induction of BTSP. In all whole-cell recordings, the series resistance was monitored to be between 10 and 40âMΩ throughout the recording.
HeLa and HEK293FT cell maintenance, transfection and imaging
HeLa cells (American Type Culture Collection, CCL-2) and HEK293FT cells (Thermo Fisher) were grown in Dulbeccoâs modified Eagle medium supplemented with 10% FBS at 37â°C in 5% CO2. Plasmids were transfected into HeLa cells using Lipofectamine 3000 (Invitrogen). Imaging was performed 24â48âh following transfection in a HEPES-buffered ACSF solution (20âmM HEPES pHâ7.3, 130âmM NaCl, 2âmM NaHCO3, 25âmM d-glucose, 2.5âmM KCl and 1.25âmM NaH2PO4) with 2âmM CaCl2 and 2âmM MgCl2 by 2pFLIM as described below. When indicated, cells were stimulated with bath application of ionomycin (Tocris Biosciences) and then EGTA.
Fluorescence-coupled size-exclusion chromatography
Expression vector DNA (2âµg) including Camuiα were transfected into HEK293S GnTI- cells (2âÃâ106 cells per well in 6-well plates) cultured in FreeStyle 293 (Thermo Fisher) using TransIT2020 transfection reagent (Mirus Bio). Cells were collected 48âh after transfection, washed with ice-cold PBS and sonicated in 250âµl TBS (20âmM Tris-HCl (pHâ8.0) and 200âmM NaCl) using a Misonix Sonicator 3000 (3 times, 30âs, power level of 9.0). The lysate was ultracentrifuged at 70,000âr.p.m. for 10âmin (TLA110 rotor). The supernatant (20âµl) was loaded onto a Superose-6 size-exclusion chromatography column (10/300 GL; GE Healthcare), pre-equilibrated with TBS, and run at a flow rate of 0.4âmlâminâ1. The eluent from the Superose-6 column was detected using a fluorometer (RF-10AXL, Shimadzu) with the following settings: excitation, 475ânm; emission, 507ânm; time increment, 0.5âs; integration time, 1âs; and recording time, 75âmin. The fluorescence-coupled size-exclusion chromatography data points were plotted using OriginPro graphic software (OriginLab v.9.5).
Fluorescence correlation spectroscopy
HEK293FT cells (Thermo Fisher) were transfected with the plasmids using Lipofectamine 3000 (Thermo Fisher) and cultured for 2âdays at 37â°C and 5% CO2. After washing the plate wells once in PBS buffer, the cells were lysed for 5âmin with M-PER mammalian protein extraction reagent (Thermo Scientific), including Halt protease inhibitor (Thermo Scientific) and 5âmM EDTA. The lysates were centrifuged at 20,000g for 10âmin and the supernatants were used for fluorescence correlation spectroscopy (FCS) measurement by diluting 2â15-fold in PBS buffer including the protease inhibitor. The FCS measurements were performed at 23â°C under a two-photon microscope without laser scanning, equipped with a Ti:Sapphire laser (Chameleon Ultra II, Coherent) tuned to a wavelength of 920ânm. The time-correlated single-photon counting data were collected for 60â120âs using a water-immersion objective (LUMPlanFL N Ã60 NA 1.0âW, Olympus) directly immersed in 300âµl of the lysate solution, a single-photon counting board (Time Harp 260, PicoQuant) and a software of TTTR mode real-time correlator in TimeHarp 260 (v.3.0). Data analysis was performed using FoCuS-point software37.
Optical CaMKII inhibition experiments
The CaMKII inhibition experiments were performed in organotypic hippocampal slices using previously described paAIP2 (ref. 12). In these experiments, slices were virally infected with 0.5â1âµl AAV mixture per slice (containing AAV9-Camk2a-Cre at 2âÃâ1012 vg per ml (1:1,000 dilution, Addgene (105558-AAV9) and rAAV8-DIO-CBA-paAIP2-mEGFP at 4.2âÃâ1012 vg per ml, UNC GTC Vector) at days in vitroâ4â6 and imaged or patched at days in vitroâ10â13. Cells with strong eGFP expression were used for experiments. Labelled cells were patched with the K+-based internal solution (see above) plus Alexa-594 dye in the patch pipette as described above. LED light stimulation (470ânm, M470L5, Thorlabs) was used to activate paAIP2.
Two-photon microscopy and 2pFLIM
Custom-built two-photon fluorescence lifetime imaging microscopy was used to perform 2pFLIM as previously described38. 2pFLIM imaging was performed using a Ti:Sapphire laser (Coherent, Chameleon or Spark Alcor 920ânm (Spark Lasers)) at a wavelength of 920ânm with a power of 1.0â1.4âmW. Fluorescence emission was collected using a water-immersion objective (Ã60, NA 0.9, Olympus), divided with a dichroic mirror (565ânm) and detected with two separated photoelectron multiplier tubes placed after the wavelength filters (Chroma, 510/70-2p for green and 620/90-2p for red). Both red and green fluorescence was detected with photoelectron multiplier tubes with a low transfer time spread (H7422P40; Hamamatsu). Photon counting for fluorescence lifetime imaging was performed using a time-correlated single-photon counting board (Time-harp 260, Pico-Quant) using custom software (https://github.com/ryoheiyasuda/FLIMage_public). 2pFLIM images were collected at 64âÃâ64 pixels at the frame rate of 7.8âHz (128âms per frame), and the time course was filtered with a moving average over 30âframes. A second Ti:Sapphire laser tuned at a wavelength of 720ânm was used to uncage MNI-caged glutamate.
Ca2+ imaging
Ca2+ imaging was performed by loading calcium dyes Cal-590 (50â100âμM, AAT Bioquest) together with a structural marker Alexa-488 (100âμM, Thermo Fisher Scientific). The Ca2+ sensor intensity measurements were collected at 64âÃâ64 pixels at the frame rate of 7.8âHz with 2pFLIM (lifetime information was not used). The Ca2+ response was calculated by normalizing the intensity with the intensity of Alexa-488. The membrane voltage was also recorded during Ca2+ imaging under the current-clamp mode. In a subset of experiments, uncaging-evoked EPSPs were measured before and after BTSP induction. For simultaneous Ca2+ and CaMKII imaging experiments, Ca2+ was normalized to the average of the first 100 frames before the induction of BTSP. For Ca2+ imaging experiments in acute hippocampal slices, we performed a whole-cell patch clamp with electrodes (4â6âMΩ) loaded with Cs+-based internal solution (see above) plus Cal-590 (50âµM). We measured the baseline Ca2+ for 2â4âmin and then applied a published BTSP protocol1, whereby Schaffer collaterals were stimulated with bipolar electrodes 10 times at 20âHz and paired with postsynaptic current injection (300âpA for 300âms) with a delay of 750âms for 5 times. Then, Ca2+ imaging was resumed for another 2â4âmins. The Ca2+ events were detected using a custom Python code, whereby 3 times the standard deviation of the baseline noise was used as a detection threshold after the subtraction of the basal trend line obtained by linear regression.
2pFLIM analysis
2pFLIM analysis was performed as previously described39. To measure the fraction of the donor that was undergoing FRET with the acceptor (binding fraction), we fit a fluorescence lifetime curve summing all pixels over an entire image with a double exponential function convolved with the Gaussian pulse response function as follows:
$$F(t)={F}_{0}[{P}_{{\rm{D}}}\,H(t,{t}_{0},{\tau }_{{\rm{D}}},{\tau }_{{\rm{G}}})+{P}_{{\rm{AD}}}\,H(t,{t}_{0},{\tau }_{{\rm{AD}}},{\tau }_{{\rm{G}}})]$$
(1)
where ÏAD is the fluorescence lifetime of the donor bound with the acceptor, PD and PAD are the fraction of free donor and donor undergoing FRET with the acceptor, respectively, and H(t) is a fluorescence lifetime curve with a single exponential function convolved with the Gaussian pulse response function:
$$H(t,\,{t}_{0},\,{t}_{{\rm{D}}},\,{t}_{{\rm{G}}})=\frac{1}{2}\exp \left(\frac{{\tau }_{{\rm{G}}}^{2}}{2{\tau }_{{\rm{D}}}^{2}}-\frac{t-{t}_{0}}{{\tau }_{D}}\right){\rm{e}}{\rm{r}}{\rm{f}}{\rm{c}}\left(\frac{{\tau }_{{\rm{G}}}^{2}-{\tau }_{{\rm{D}}}(t-{t}_{0})}{\surd 2{\tau }_{{\rm{D}}}{\tau }_{{\rm{G}}}}\right)$$
(2)
in which ÏD is the fluorescence lifetime of the free donor, ÏG is the width of the Gaussian pulse response function, F0 is the peak fluorescence before convolution and t0 is the time offset, and erfc is the complementary error function.
To generate the fluorescence lifetime image, we calculated the mean photon arrival time, <t>, in each pixel as follows:
$$ < t > \,=\int tF(t){\rm{d}}t/\int F(t){\rm{d}}t,$$
Then, the mean photon arrival time was related to the mean fluorescence lifetime, <Ï>, by an offset arrival time, t0, which was obtained by fitting the entire image as follows:
$$ < \tau > = < t > -{t}_{0}.$$
For analysing fluorescence lifetime in regions of interests (ROIs) (spines or dendrites), we calculated the fluorescence lifetime by fitting the decay curve with equation (1), assuming ÏD, ÏAD, ÏG and t0 are constants within each image session. To measure the CaMKII time of occurrence and peak lifetime change in BTSP and control experiments, the raw traces were first normalized using the first 100 frames as baseline and then the normalized data were smoothened using a moving average of 60 data points. Following this processing, the time of CaMKII peak and amplitude was manually calculated on individual CaMKII traces.
Statistics and reproducibility
All values are presented as the meanâ±âs.e.m. unless otherwise noted. The number of independent measurements or cells (n) is indicated in figures or figure legends. For electrophysiology experiments, the recordings were performed on 9â26 neurons (1 neuron per slice) from at least 2 different litters. For imaging experiments, the experiments were independently performed on 12â82 dendrites from at least 5 neurons (1 neuron per slice) from at least 2 different litters. For pharmacology experiments, 1â2 control experiments were performed on the same slices before the specific drug was added to the ACSF. In experiments for which DMSO was used as a vehicle, we performed control experiments on different days but on the slices made from the same batch as used in the experiments. Unpaired two-tailed Studentâs t-test was used to compare two independent samples. Paired two-tailed Studentâs t-test was used to compare dependent variables (beforeâafter, somaâdendrite). One-way ANOVA followed by Dunnettâs multiple comparison test was used to compare more than two independent samples. Two-way ANOVA followed by Dunnettâs or Tukeyâs multiple comparison test was used to compare grouped datasets. Correlation analysis was done by computing Pearson correlation coefficients. Data were organized in Microsoft Excel (v.2016). Data smoothening, statistical tests and Pâvalues are noted in each figure legend and were computed using GraphPad Prism (v.7.03, 9.5). Schematics of Figs. 1a, 3a and 5a and Extended Data Fig. 13 were created using Microsoft PowerPoint (v.2016) and Adobe Illustrator (v.27.9.1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.