Credit: Simon Prades
Will a human ever celebrate a 150th birthday? You bet, says Stephen Austad, a biology of ageing researcher at the University of Alabama at Birmingham. Austad is so sure that life expectancy is poised for another rapid rise, he put money down in 2000 that the first person to reach 150 was already alive.
It’s a fantastical idea, counters Jay Olshansky, a longevity researcher at the University of Illinois Chicago, who took Austad’s bet and foresees a payout for his descendants in 2150.
So far, the numbers seem to fall in Olshansky’s favour. During the twentieth century, medical advances added 30 years to human life expectancy. Since then, the rise has slowed significantly — as Olshansky was sure it would.
Nature Index 2025 Ageing
“In 1990, colleagues and I forecast that the rise in life expectancy will slow, as more people live long enough to be exposed to the immutable force of ageing,” Olshansky says1. When Olshansky revisited this analysis in 2024, he and his co-authors confirmed that life expectancy in the developed world was flatlining2.
The human body has been pushed about as far as it can go by conventional medicine, Olshansky says. “As long as ageing remains unmodified, you can’t push out the envelope of survival much beyond where we are today.” The only path to further radical human lifespan extension, he concluded, is to slow the ageing process itself.
Austad agrees. “I think we’re very close to being able to do it,” he adds, noting numerous approaches that have shown promise in animals and, increasingly, in people.
Perhaps surprisingly, given their wager, Olshansky also sees great potential in this work. “I’m very optimistic that we will be able to slow down the biological process of ageing,” he says. “I think it will happen in our lifetime.” On how much this might impact lifespan, Olshansky and Austad differ, although both agree that such considerations are a distraction. Ultimately, the point is not how long we live, but how well we live.
Young of heart
Living longer is a secondary goal of geroscience, a fast-growing field focused on uncovering the biological changes that make our cells, tissues and organs more prone to conditions such as cancer, cardiovascular disease and neurodegenerative disorders as we age. Targeting individual diseases might extend lifespan, but it no longer guarantees a longer ‘healthspan’ — years lived fit and well, Austad says. For instance, age-adjusted deaths from heart disease have fallen by one-third since 2000, but people who don’t die from it typically never fully recover, compromising their quality of life and burdening health-care systems.
“The fear is, if we continue with the disease model, we’re going to live longer and longer in the ‘red zone’ — with frailty and disability,” Olshansky says.
Geroscience resets age-related disease management by targeting the biggest risk factor for their onset: ageing itself. One line of research has been to study centenarians who have remained naturally fit and healthy far beyond average life expectancy. “As a clinician, I was always intrigued by individuals who are very old and functioning very well” and finding out “what stimulates the longevity and healthspan in these individuals”, says Andrea Maier, a healthy-ageing researcher at the National University of Singapore.
Other leads have come from the observation, first made in rodents a century ago, that animals fed a heavily calorie-restricted diet live longer. “In mice, it works, it slows down ageing,” says Zahida Sultanova, a postdoc at the University of East Anglia, UK, who studies factors that affect ageing. Studying how cellular processes changed in these long-lived animals has delivered many insights into ageing.
Several processes identified in calorie-restricted animals have also shown up in healthy human centenarians. One such mechanism, identified by biology of ageing researcher, Leonard Guarente, and colleagues at the Massachusetts Institute of Technology in Cambridge, pointed to the importance of a set of genes and associated proteins called sirtuins3. Many organisms are now known to possess them. “If you make them more active, you extend the lifespan,” Guarente says. Some centenarians also carry extra-active sirtuins. Sirtuins were found to play a role in DNA maintenance and repair, but they rely on a molecule called NAD+, which naturally declines with age. In ageing mice, dietary supplements that increase cellular NAD+ levels can boost sirtuin activity and extend healthspan and lifespan.
Guarente has spun off a company from his research to test this concept in people. “You can’t realistically run a clinical trial to determine how long humans live, because you’d have to wait 50 years for the result. So, we look at surrogate markers of health,” Guarente says. So far, in early-stage trials, boosting NAD+ has safely lowered markers of inflammation thought to be at the core of conditions including non-alcoholic fatty liver syndrome4 and chronic obstructive pulmonary disease5.
Dousing inflammation
Chronic inflammation is so closely associated with ageing that it has been dubbed ‘inflammageing’. This over-activation of the immune system involves a complex interplay of signalling molecules called cytokines, some promoting inflammation and some suppressing it.
Cytokine interleukin-11 (IL-11) was long thought to be anti-inflammatory until, in 2017, Stuart Cook from Duke-National University of Singapore Medical School and his colleagues showed the opposite was correct6.
“We found that IL-11 was involved in multiple ageing processes,” says Cook, a clinician scientist researching new biomedical targets for healthspan. Following a chance observation that IL-11 levels were increased in the organs of old rats, they investigated further. In a mouse study7 published in 2024, the team showed that treating the animals with an antibody that binds IL-11 and blocks its effects extended lifespan by up to 25%. “The benefits of anti-IL-11 were apparent in every organ we looked at,” says Cook, who suspects IL-11 is an evolutionary appendage that once played a role in limb regeneration in ancient fish but has no beneficial function in humans.
Clinical trials of IL-11-inhibitors have already begun. In June 2025, Alphabet-owned Calico Life Sciences struck a licensing deal for one such IL-11 blocker, developed by Chinese biotech firm Mabwell, based in Shanghai. Some of the big companies collaborating with Calico — including US pharma company AbbVie — should enable large-scale clinical trials to test IL-11 inhibition in people, Cook says. “Then we will know once and for all if anti-IL-11 is an anti-ageing drug for humans.”
Cleaner living
Of course, there’s no guarantee that any potential anti-ageing medicine that generates results in mouse studies will succeed in human clinical trials. In the anti-ageing field, a group of drugs called senolytics have not, so far, fulfilled their preclinical promise.
Senolytics are designed to clean out dysfunctional ‘senescent’ cells, which accumulate in many tissues and cause inflammation, prompting the hypothesis that such cells could be a fundamental driver of ageing. Following animal studies looking at the potential healthspan and lifespan benefits of senolytics, multiple clinical trials have been launched in the past five years — but so far, have failed to show the anticipated benefits in people, Austad says. “Senolytics went from the laboratory to clinical trials extremely fast — we’ll see if they fall out of favour just as fast,” he says.
For Austad, one reason for the poor success rate in translating animal-study successes into human findings is the way animal studies are conducted. Lab animals are cosseted, kept continually warm, well-fed and protected from pathogens. “This artificial situation has completely misled us about how treatments are going to translate into the real world,” says Austad, who is testing anti-ageing interventions in mice kept under a range of conditions to better approximate real life. “Treatments that work under all those conditions are the things that we should be most interested in,” he says.
Reuse and recycle
Several anti-ageing approaches repurpose existing drugs, rather than looking for new ones. Two have been examined for their ability to mimic the metabolic effects of calorie restriction. One is the widely prescribed blood-glucose-lowering diabetes drug, metformin. Another is rapamycin, an immunosuppressant that inhibits a cellular nutrient sensor controlling cell growth and repair. Blocking this receptor can mimic caloric restriction by tricking the body into thinking food is scarce, Sultanova says. “You eat normally but hide it from yourself.”
In 2024, Sultanova and her colleagues released a meta-analysis of 167 animal studies8, looking at the lifespan impacts of rapamycin and metformin compared with actual caloric restriction. The analysis found that caloric restriction gave the largest and most consistent lifespan extension, closely followed by rapamycin. Metformin showed no clear benefit.
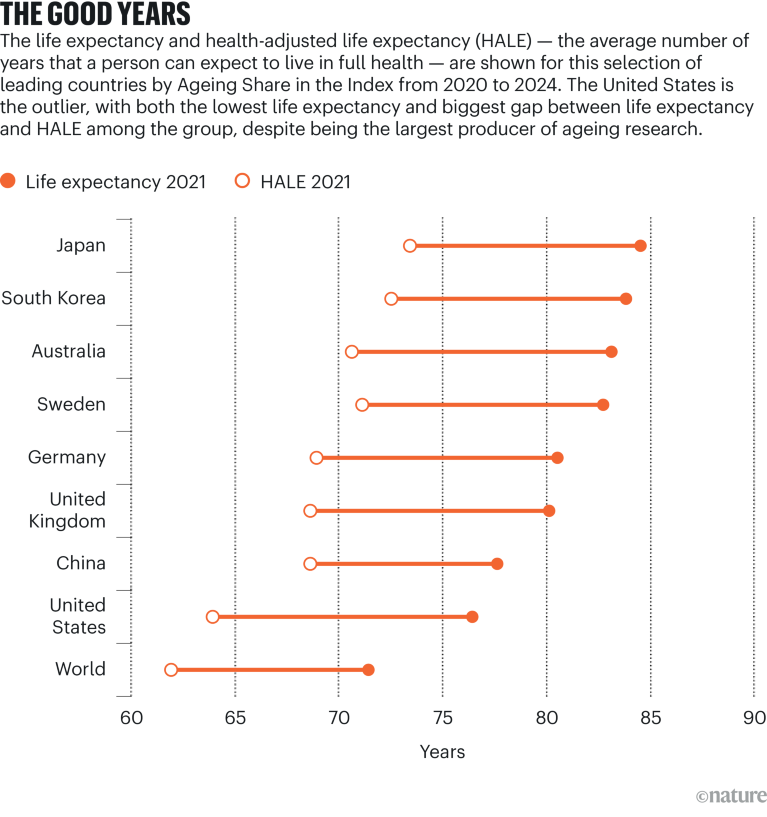
Source: World Health Organization