Plant materials and growth conditions
The rice (O. sativa L.) varieties Zhonghua 11 (ZH11), Xiushui 11 (XS11), IRBB7, Wuyujing 3 (WYJ3), IR64 and Wuyunjing 8 (WYJ8) were used as wild type. Tobacco (N. tabacum cv. Samsun–NN) and maize (Zea mays cv. B104) were used as WT. A. thaliana, B. napus, Glycine max, Pinus elliottii, Ginkgo biloba, Capsicum annuum, Solanum melongena, Populus canadensis, Chlamydomonas reinhardtii, Marchantia polymorpha, Sphagnum palustre, Selaginella moellendorffii, Nymphaea colorata and Salix babylonica were collected from growth chambers or the field for isotope-labelling feeding experiments. The plants were grown in the growth chamber with a 12-h light (28 °C):12-h dark (22 °C) photoperiod, 500–600 μmol m−2 s−1 light intensity and 50% humidity. In the field experiments, the plants were grown under a conventional cultivation environment in a paddy field of the Botany Garden of Zhejiang Normal University in Jinhua (119° 63′ E, 29° 130′ N), China.
EMS mutagenesis and forward genetic screen
The seeds of wild-type plants were mutagenized by EMS as described47. The mutants were screened from the M2 seedlings following a modified method based on a bacterial biosensor, Acinetobacter sp. ADPWH_lux36,37. In brief, ~0.05 g young leaves of 3-week plants were collected and placed into a well of 2-ml 96-well plates containing 600 μl LB. Then the samples were incubated in the water bath at 95 °C for 30 min. After the samples were cooled to room temperature, 50 μl of leaf extract was successfully transferred to a new black 96-well cell culture plate, and 50-μl culture of the biosensor strain Acinetobacter sp. ADPWH_lux (OD600 = 0.4) was added and mixed. The plates were incubated at 37 °C for 90 min, and the luminescence was read using Infinite 2000 PRO (Tecan).
Map-based cloning and bulk population sequencing
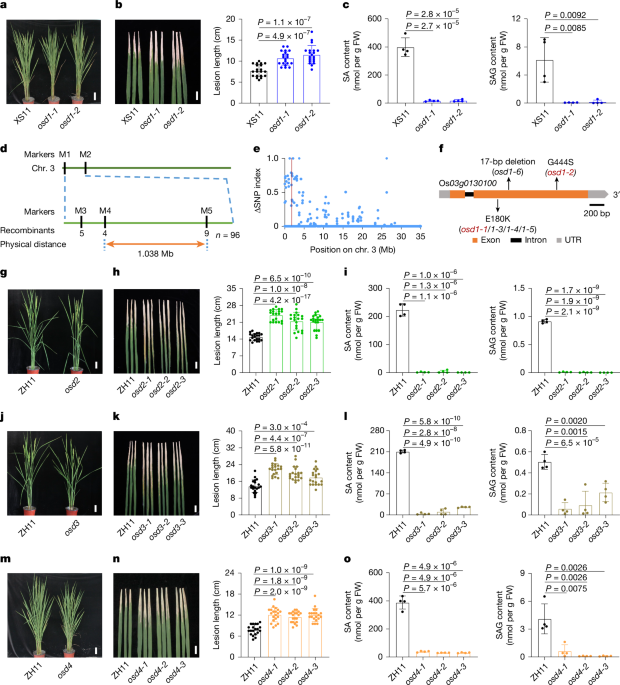
F1 generation plants were obtained by crossing osd1-1 with IR64 rice variety. All individual plants with low SA content in the F2 population were selected for DNA extraction. The simple sequence repeats (SSR) and sequence-tagged-site (STS) markers were screened for polymorphic markers. The PCR products were separated by 5% agarose gel electrophoresis. All the primers used in this study are listed in Supplementary Table 3.
For bulk population sequencing, an equal amount of DNA was extracted from four DNA pools, the osd1-1-type pool (44 lines) and the WT-type pool (50 lines) randomly selected from the BC1F2 individuals of osd1-1 and XS11, the parent WT pool (50 lines), and the osd1-1 mutant pool (50 lines). The library was prepared with the Illumina TruSeq DNA PCR-free prep kit and sequenced using the Illumina HiSeq X-ten platform. To identify the mutation site, we mapped the reads to the rice reference genome using BWA-MEM (v.0.7.17) with the default parameters. Alignments were sorted with SAM tools (v.1.6) and duplicates were marked with Picard Tools (v.2.27.5+dfsg). SNPs were called with SAM tools (v.1.6)/BCF tools (v.1.5)48. To reduce false-positive detection of SNPs, SNP positions with a SNP quality score. In brief, the ratio between the number of reads of a mutant SNP and the total number of reads covering the SNP site was defined as the SNP index. The ΔSNP index is defined by subtracting the SNP index value of the WT-type pool from the osd1-1-type pool. The average of ΔSNP index was calculated using a sliding-window approach with a 25-kb window size and a step size of 5 kb, and plotted across the 12 rice chromosomes.
Quantification of SA, SAG, BB and BS in plants
SA and SAG in plants were extracted and quantified as described49. SA, SAG, BB and BS contents shown in Extended Data Fig. 8a–c were extracted as described49 with some modification. In brief, ~50 mg of leaf tissue was collected and flash frozen in liquid nitrogen, finely ground with freezing grinder and extracted with 500 μl methanol with internal standards (D6-SA, D-1156, C/D/N ISOTopes) at 4 °C for 4 h. After centrifugation, 150 μl supernatant was taken out for quantification of BB and BS by the high-resolution gas chromatography–mass spectrometry system (HRGC–MS, Thermo Fisher Scientific) which consisted of a Trace1610 series GC, an AS 1610 Liquid Autosampler and an Exactive GC Orbitrap MS analyser with electron ionization. The remaining mixture was subsequently extracted twice with 1 ml 80% methanol and 500-μl 100% methanol at 4 °C for 4 h. After centrifugation, the supernatant was collected and dried by nitrogen gas. Then the residue was resolved in 300 μl of 30% methanol for quantification of SA and SAG by the ExionLC (AB SCIEX) high-performance liquid chromatography (HPLC) instrument paired with a QTRAP 5500 mass spectrometer (AB SCIEX).
CRISPR–Cas9 gene editing and gene overexpression
The osd2 and osd3 mutants in the ZH11 background were obtained from BIOGLE GeneTech (http://biogle.cn). The osd4 mutants in XS11 background and oskat1 kat2 double mutants in ZH11 background were generated by CRISPR–Cas9 genome editing technology as described50. The tobacco nsd1 and nsd3 mutants were generated by CRISPR–Cas9 genome editing technology as described51,52. The maize zsd3 double mutants were generated from wild-type B104 by Wuhan EDGENE Biotechnology. The mutation of CRISPR-mediated mutants was confirmed by DNA sequencing.
The coding sequences (CDS) of OSD1, OsKAT2, OSD2, OSD3 and OSD4 genes were amplified by PCR and cloned into pCR8 (K250020, Thermo Fisher Scientific). Then the constructed entry vectors were cloned into the binary vector pUbi-pMDC32 or pMDC43 to construct pUbi::OSD1, 35S::GFP-OsKAT2, pUbi::OSD2, 35S::GFP-OSD3, and 35S::GFP-OSD4 vectors by the Gateway LR Clonase II enzyme mix (Thermo Fisher Scientific, USA) or ClonExpress II One Step Cloning Kit (Vazyme Biotech, China), respectively. Binary vectors were transformed into rice by Agrobacterium tumefaciens-mediated transformation.
Pathogen test and trypan blue staining
Rice plants grown in paddy fields were inoculated with X. oryzae pv. oryzae (Xoo) Philippine strain P6 (PXO99A) at the tillering stage following a leaf-clipping method as described53,54. Xoo was cultured on agar medium that contained 20 g sucrose, 5 g peptone, 0.5 g Ca(NO3)2, 0.43 g Na2HPO4 and 0.05 g FeSO4 per litre and were cultured at 28 °C for 2–3 days. The culture was resuspended with sterile water to the optical density at 600 nm (OD600) = 1.0 and immediately used for plant inoculation. The infected symptoms were photographed and measured at 14 days post-inoculation.
The fully expanded leaves from rice plants at the tillering stages were used for syringe infiltration to observe the hypersensitive reaction (HR) following a described method55. In brief, bacterial suspensions of optical density of OD600 = 0.5 were used for syringe infiltration. The plants were grown in the growth chamber with a 12-h light (30 °C):12-h dark (28 °C) photoperiod, 500–600 μmol m−2 s−1 light intensity, and 80% humidity 7 days before infiltration. Samples were taken before and 48 h after infiltration to analyse the content of SA, SAG, BB and BS. Trypan blue staining of the HR reactions was performed 3 dpi following a previously described method56. The phenotype of the HR reactions was observed at 4 dpi. The images were captured by using a stereomicroscope (SteREO Discovery.V12, Carl Zeiss Microscopy).
Transcriptional analysis of gene expression
Total RNAs were extracted with the Trizol reagent (Aidlab) from different tissues of the 70-day-old ZH11 plants. The 4-cm leaf truncation below the cut edge was collected at 12, 24, 48 and 72 h post-inoculation of Xoo. RT–qPCR was performed using SYBR Green (Q712, Vazyme Biotech) on the QuantStudio 1 Real-Time PCR Thermal Cycler (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Protein subcellular localization
To generate pCR8-AIM1 and pCR8-OsKAT1, the CDS of AIM1 and OsKAT1 were amplified and inserted into pCR8. Then, the constructed entry vectors of OSD1–4, OsKAT1/KAT2 were cloned into the binary vector pSAT6 or pMDC43 to construct GFP fusion expression vectors. The pCR8-AIM1 was cloned into the binary vector mCherry-pSAT6 to construct 35S::mCherry-OsAIM1 vector as a peroxisome-localized marker.
The rice protoplasts were prepared and transformed with expression vectors following a previously described method with some modification57. In brief, the stem and sheath tissues from rice seedlings (2 to 3 weeks old) were cut into approximately 0.5 mm strips. The strips were immediately transferred into 0.6 M mannitol for quick plasmolysis treatment, followed by enzymatic digestion in the dark with gentle shaking. The protoplasts were collected by filtration through 40 μm nylon meshes. After transfection with the vector by using the PEG-mediated transfection approach, the protoplasts were incubated at 22 °C for 10 h. The fluorescence images were observed by the Zeiss LSM 880 Confocal Microscope system (Carl Zeiss Microscopy) using an excitation 488-nm laser with an emission wavelength of 505–550 nm for GFP, a 561-nm laser with an emission wavelength of 600–660 nm for mCherry, and a 488-nm laser with an emission wavelength of 650–710 nm for chloroplast. mCherry–AIM123 and mCherry–HDEL41 were used as peroxisome and endoplasmic reticulum-localized markers, respectively.
Endoplasmic reticulum membrane preparation was carried out as previously described58. Immunoblots were probed with antibodies against GFP (Invitrogen A6455, 1:3,000), A. thaliana fructose-1,6-bisphosphatase (PhytoAB, PHY3095A, 1:3,000), or A. thaliana heat shock 70 kDa protein BIP1/2 (PhytoAB, PHY1481A, 1:1,000), and goat anti-rabbit IgG antibody (PhytoAB, PHY6000, 1:1,000), according to standard procedures.
Heterologous expression and purification of recombinant proteins
The CDSs of different genes were amplified by PCR using gene-specific primers from rice cDNAs, and cloned into pMAL-c2X (New England BioLabs). The PhCHD gene was synthesized and cloned into pMAL-c5X (New England BioLabs) by Beijing Tsingke Biotech. The correctly sequenced plasmids were transformed into E. coli BL21 (DE3, pLys3; Invitrogen) or Rosetta 2 (DE3, Beyotime). The culture was induced for protein expression with 0.6 mM IPTG, and then incubated for 24 h at 18 °C. The recombinant protein was purified according to the manufacturer’s protocol of Amylose Resin (New England BioLabs).
The CDS of OSD3 was amplified from the pCR8-OSD3 and cloned into pESC-URA vector (Agilent Technologies) to generate Flag–OSD3 fusion protein expression vector using Clonexpress II One Step Cloning Kit. The plasmid was transformed into yeast (S. cerevisiae) strain WAT11. Yeast cells carrying pESC-URA or pESC-URA-OSD3 were cultured in 5 l of medium and induced by 2% galactose. Microsomal proteins were purified according to a previously described method59. The protein concentration was determined by the Bradford assay60. The immunoblots were probed with antibody against Flag (F3165, Sigma-Aldrich, 1:1,000), and goat anti-mouse IgG antibody (BS12478, Bioworld, 1:1,000), according to standard procedures.
In vitro biochemical enzyme assays
The OSD1 enzyme assay was performed as described30,38. The reaction mixture contained 100 mM Tris-HCl buffer (pH 7.5), 2.5 mM MgCl2, 1 mM ATP (D7378, Beyotime), 0.8 mM CoA (ST353, Beyotime), 0.3 mM trans-CA and around 0.45 μg μl−1 MBP–OSD1 protein. The mixture was incubated at 25 °C for 30 min and then terminated by boiling for 3 min. The enzyme activity was calculated by using the trans-CA consumption rate.
The AIM1 enzyme activities were assayed following a method with modification25. The reaction mixture contained 100 mM Tris-HCl buffer (pH 7.5), 2.5 mM MgCl2, 50 mM KCl, 0.5 mM CA-CoA, 1 mM pyruvic acid, 1 mM NAD+, 2 units of lactate dehydrogenase, and 0.185 μg μl−1 MBP–AIM1 protein. The mixture was incubated at 30 °C for 30 min and terminated by boiling for 3 min. The MBP–PhCHD enzyme was used as a positive control25.
The conditions for AIM1 and OsKAT coupling enzyme assays were developed according to the literature25,61. The reaction mixture contained 100 mM Tris-HCl buffer (pH 7.5), 2.5 mM MgCl2, 50 mM KCl, 0.5 mM CA-CoA, 2 mM CoA, 1 mM pyruvic acid, 1 mM NAD+, 2 units of lactate dehydrogenase, 0.075 μg μl−1 AIM1 protein and 0.112 μg μl−1 OsKAT1 protein or 0.065 μg μl−1 OsKAT2 protein. The mixture was incubated at 30 °C for 20 min and terminated by boiling for 3 min.
The enzyme assay of OSD2 was developed according to the literature44,45. The reaction mixture contained 200 mM phosphate buffer (pH 6.0), 300 mM NaCl, 1 mM dithiothreitol, 0.4 mM BA-CoA, 0.5 mM BAlc (Boer) and 0.25 μg μl−1 OSD2 protein. The mixture was incubated at 10 °C for 30 min. All reactions were stopped by adding an equal volume of 100% methanol to denature the protein. After centrifugation at 13,000g for 10 min, the supernatant was air-dried with nitrogen gas and then resuspended with 300 μl methanol.
The OSD3 activities were assayed essentially as described previously62, with some modifications. The reaction mixture (500 μl) containing 80 mM sodium phosphate (pH 7.0), 5 mM DTT, 5 mM NADPH (ST360, Beyotime), 50 μM BB (RHAWN), and 2.5 μg μl−1 microsomal proteins was incubated at 30 °C for 1 h. All the reaction was terminated by the addition of 100 μl acetic acid. The mixed solution was mixed with 1 ml water statured ethyl acetate, and centrifuged at 13,000g for 10 min.
The enzyme assay of OSD4 was developed according to the literature63. The reaction mixture contained 100 mM Tris-HCl buffer (pH 8.0), 0.6 mM BS (Aladdin), and 0.48 μg μl−1 OSD4 protein. The mixture was incubated at 30 °C for 30 min. The reaction was stopped with an equal volume of acetonitrile.
For substrate specificities of the enzymes, the compounds from related biosynthetic pathways or structurally similar substrates were tested under optimal reaction conditions. For kinetic analysis, an appropriate enzyme concentration and incubation time were chosen. All the supernatants of enzyme reaction products were filtered by 0.22-μm membrane filter and analysed by LC–MS, LC–UV–MS or GC–MS. The enzyme kinetic parameters were determined by the Michaelis–Menten equation for OSD1 and OSD4 or the allosteric sigmoidal enzyme kinetics equation for OSD2 by using GraphPad Prism (v.9.3.1).
LC–MS and LC–UV–MS analysis
The chromatographic peak retention time and DAD spectrum of enzymatic products were analysed by the ExionLC (AB SCIEX), which consisted of a controller, an AD autosampler, two AD pumps, an AD column oven, and a photo-diode array detector. Detailed conditions are shown in Supplementary Table 4. The mass spectra of the enzymatic products of OSD1, AIM1 and AIM1 coupling with OsKAT1/KAT2, OSD3 and OSD4 were characterized by a TripleTOF 4600 mass analyser (AB SCIEX) paired with the Nexera X2 HPLC System (Shimadzu). The TripleTOF 4600 mass analyser was equipped with electrospray ionization (ESI). The Nexera X2 HPLC instrument consisted of a DGU-20A degasser, a SIL-30AC autosampler, two LC-30AD pumps, a CTO-20AC column Oven, and an SPD-20A detector. Detailed conditions are shown in Supplementary Tables 5 and 6.
The SA and its derivatives were quantified by the ExionLC (AB SCIEX) paired with a QTRAP 5500 mass spectrometer (AB SCIEX). The QTRAP 5500 mass spectrometer was equipped with an electrospray ionization interface (ESI, Turbo V). The multiple reaction monitoring (MRM) mode was used, and the mobile phase, flow programme, and the specific precursor ion-to-product ion transitions of all target compounds with the detailed conditions were described in Supplementary Table 7. The SA and SAG were accurately quantified using internal standards, and the concentration of other compounds was calculated according to the standard curve of the standards. Both data acquisition and instrument control were coordinated by Analyst Software (v.1.6.3).
GC–MS analysis
The GC–MS system (a 7890B GC coupled with 5977B mass spectrometer detector, Agilent Technologies) was used for BB content quantification in OSD2 enzyme assay. The detailed conditions are shown in Supplementary Table 8, and the concentration of BB was quantified according to the standard curve.
The HRGC–MS system (a Trace1610 series GC coupled to an Exactive GC Orbitrap mass analyser, Thermo Fisher) was used to characterize the mass spectra of OSD2 enzymatic products and quantify the BB and BS in plant samples according to the standard curve. The detailed conditions are shown in Supplementary Table 8.
Inference of homologues of key components in PAL-SA pathway
To identify the closely related homologues of key components in PAL-SA pathway of rice, a total of 25 plant species with high-quality genomes from representative taxonomic groups (Rhodophyta, Chlorophyta, Streptophyte algae, Charophyta, Bryophyta, Lycophyta, Monilophyta, Gymnospermae, Basal angiosperms, Monocots and Eudicots) in the plant kingdom were downloaded from public database, including EnsemblPlants, FigShare, FernBase, GinkgoDB, Nicomics, ORCAE, Phytozome 13 and TreeGenes (Supplementary Table 9). Whole protein sequences from the above 25 species genomes with the longest transcripts were retained as representative isoforms. To obtain high-quality protein sequences, we removed the possibly misannotated peptides with starting amino acids other than methionine and sequences containing unknown amino acid X using an in-house script. Then, STRIDE was used to infer the species tree based on the identified orthogroups64. For nodes in inferred species with low support rate (bootstrap values < 90), we correct the phylogenetic relationship among these species according to the related literatures65,66. Based on the corrected species, the closely related homologues of these key components (OSD1, AIM1, OsKAT1, OsKAT2, OSD2, OSD3 and OSD4) were identified using Orthofinder 2.5.567. The conserved protein PFAM domains for these putative homologues were identified by InterProScan (v.5.69-101.0)68, PF00501 and PF13193 for OSD1 (Os03g0130100), PF00378, PF02737 and PF00725 for AIM1 (Os02g0274100), PF00108 and PF02803 for OsKAT1 (Os02g0817700) and OsKAT2 (Os10g0457600), PF02458 for OSD2 (Os10g0503300), PF00067 for OSD3 (Os09g0441400) and PF07859 for OSD4 (Os05g0410200), respectively. The retained protein sequences with at least one conserved domain (Supplementary Table 10) were then used for multiple sequence alignment with MAFFT v7.52669 and construction of maximum-likelihood gene trees with 500 bootstrap replicates and optimal model using RAxML (v.8.2.12)70. Final gene trees for each component were constructed after removing protein sequences with extremely long branch (Extended Data Fig. 9). The retained protein sequence sets were considered as the closely related homologues of these PAL-SA pathway enzymes in rice (Supplementary Table 11).
Substrate/intermediate feeding and isotope-labelling tracer experiments
For substrate/intermediate feeding, 200 μM CA, BB, BS, or SA were separately prepared in a water solution containing 0.1% Tween-20. The WT and SA-deficient mutants at the tillering stage were applied foliar spray with the solution twice a day (for six days) before further analysis.
The isotope-labelling tracer experiments were followed a previously described method with modification46. Leaves from different plants were cut and incubated in water with 200 μM ring-13C-labelled phenylalanine (13C6-Phe, Cambridge Isotope Laboratories) for 72 h. SA, SAG, 13C6-SA, and 13C6-SAG were extracted from the samples and analysed as described previously49.
Statistical analysis
Statistical significance was determined using two-sided Student’s t-tests or one-way ANOVA with LSD test for multiple groups (≥3) of data. Statistical analysis was performed using GraphPad Prism 9.3 or IBM SPSS Statistics 21. Detailed statistical analyses are explained in the figure legends, and P values are indicated in the figures or the source data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.