You have full access to this article via your institution.
Pluripotent stem cells have special properties: they can proliferate almost indefinitely and have the capacity to differentiate into any cell that makes up an individual. These cells are found naturally in embryos, but can also be artificially induced from certain adult cells. Under the right conditions, both embryonic stem cells and induced pluripotent stem cells can be driven to differentiate into various types of cell, and efforts to produce differentiated cells from human pluripotent stem cells for clinical use have gathered pace over the past few decades. Writing in Nature, Sawamoto et al.1 and Tabar et al.2 report on clinical trials that harness pluripotent stem cells for the possible treatment of Parkinson’s disease, the second most common neurodegenerative disease worldwide.
Read the paper: Phase I/II trial of iPS-cell-derived dopaminergic cells for Parkinson’s disease
As of December, there were 115 clinical trials under way for 83 products derived from pluripotent stem cells3. Treatments for Parkinson’s disease are at a more advanced stage of development than are those for any other central nervous system disease. Parkinson’s is characterized by the progressive loss of neurons that release the neurotransmitter molecule dopamine. The motor symptoms associated with Parkinson’s disease, such as muscle rigidity, slow movement, tremor and disturbances to gait, are caused by the depletion of neurons in a region of the midbrain called the substantia nigra.
The first choice of treatment is the administration of levodopa, a precursor to dopamine that is readily transported from the bloodstream into the brain. However, as Parkinson’s disease progresses, levodopa treatment becomes less effective because there are fewer dopamine-releasing (dopaminergic) neurons available to convert levodopa into dopamine. Replenishing the dopaminergic neurons themselves through regenerative medicine could offer an alternative route to effective treatment.
Read the paper: Phase I trial of hES-cell-derived dopaminergic neurons for Parkinson’s disease
This approach was pioneered in the 1980s: researchers took cells from the fetal ventral midbrain, which is thought to be rich in dopaminergic neurons, and transplanted them into the striatum (a region to which midbrain neurons send projections) of individuals with Parkinson’s disease4. Follow-up studies5–7 showed that the transplanted area recovered its dopamine content and motor function improved, and the effects persisted for many years after transplantation.
These experiments are thought to have provided the first proof-of-concept for cell transplantation therapy for Parkinson’s disease. However, the large amount of fetal brain tissue that was needed to treat a single patient raised ethical concerns8, and the technical difficulties of obtaining the correct quantity and quality of cells for transplantation prevented the method from being widely implemented.
One solution to the ethical issues associated with the use of fetal tissue is to generate dopaminergic neurons using stem cells that can be proliferated on a large scale. Many research groups around the world have successfully produced dopaminergic neurons from human pluripotent stem cells and transplanted them into animal models of Parkinson’s disease, resulting in functional recovery9. In 2020, researchers reported the first clinical application of induced pluripotent stem cells to treat Parkinson’s disease, which involved an individual receiving midbrain dopaminergic progenitor cells (the precursors to mature neurons) that had been created from their own skin cells10.
However, the study involved only one person, meaning the results cannot be generalized, and because the study lacked a control group, the possibility of a placebo effect cannot be ruled out. Since then, methods to produce clinical-grade dopaminergic progenitor cells from both induced pluripotent stem cells and embryonic stem cells have improved, and clinical trials have been conducted (Fig. 1).
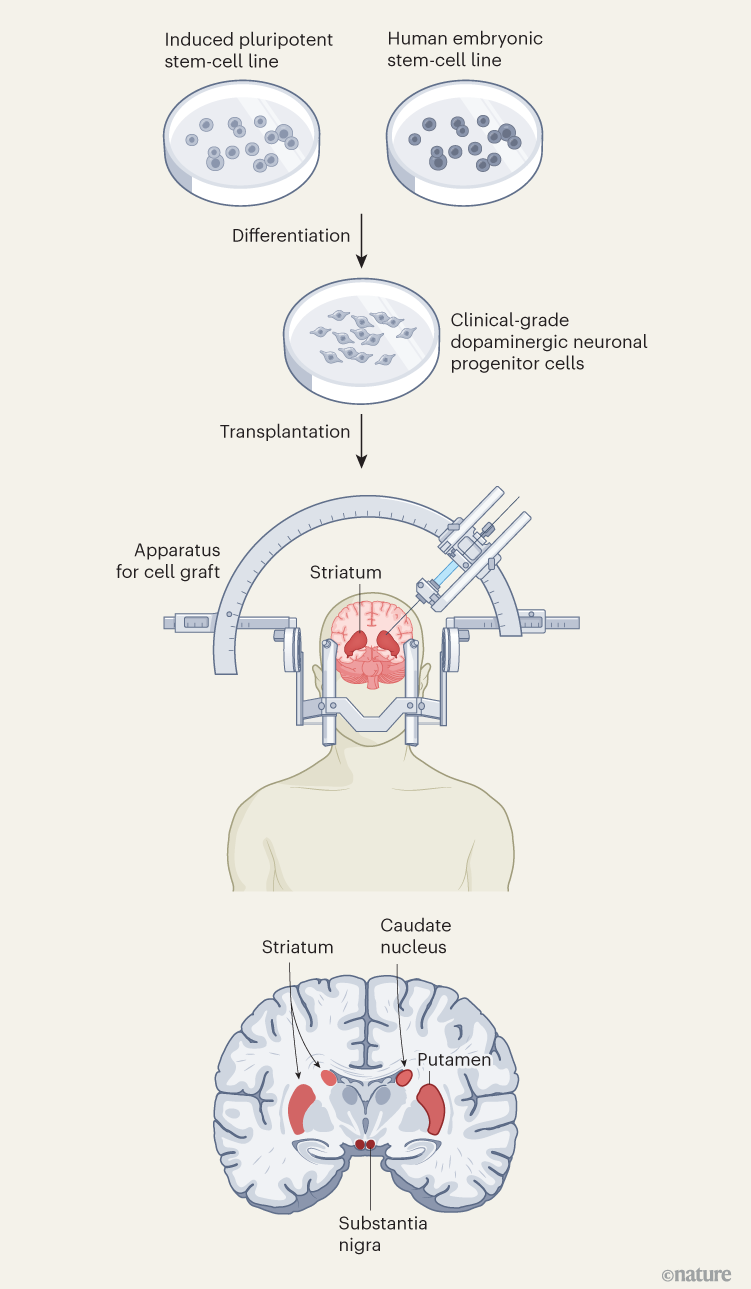
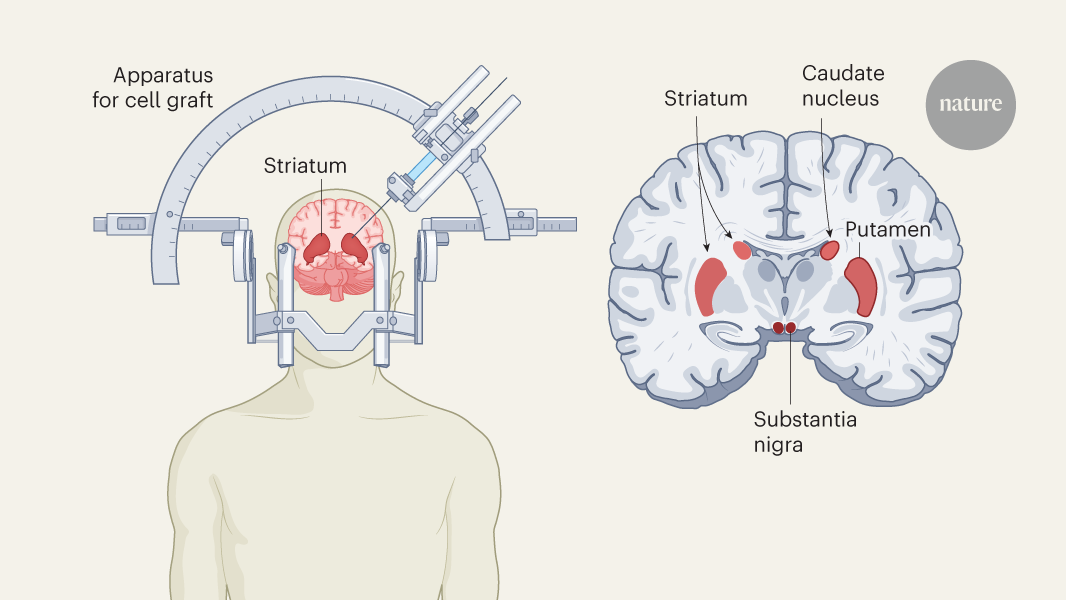
Figure 1 | Stem-cell therapy for Parkinson’s disease. Parkinson’s disease is caused by a progressive loss of dopamine-releasing (dopaminergic) neurons in the brain. Clinical trials of a stem-cell-based treatment were carried out by Sawamoto and colleagues1 and Tabar and colleagues2. Both teams used clinical-grade pluripotent stem cells, which can divide indefinitely and differentiate into any cell type, to replenish dopaminergic neurons. Sawamoto et al. used an induced pluripotent stem-cell line derived from cells from healthy adult donors, whereas Tabar et al. used a human embryonic stem-cell line derived from early embryos. Stem cells were cultured so that they formed dopaminergic neuronal progenitor cells. These cells were grafted into a brain region called the putamen, which, together with the caudate nucleus, forms the striatum. The striatum connects to the substantia nigra, where dopaminergic neuronal loss is most severe. The early (phase I/II) clinical trials mainly confirmed that the treatment is safe, but also gave some indication that it is effective at improving symptoms.
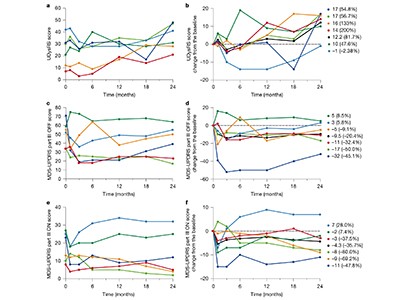
Sawamoto et al.1 report the results of a phase I/II trial conducted in Japan. Dopaminergic precursor cells, derived from an induced pluripotent stem-cell line11, were transplanted into both sides of the brain in seven people with Parkinson’s disease. The main focus of the trial was to examine safety and look for adverse events, but the authors also kept track of changes in motor symptoms and dopamine production. No serious adverse events were reported during the study period, and the transplanted cells produced dopamine without forming tumours — a serious risk associated with stem-cell therapy. The authors also observed a decrease in motor symptoms: while the participants were taking their standard medication, five out of six participants included in the efficacy analysis showed improvement in motor function, and when they stopped taking it four still showed improvement.
‘Big leap’ for Parkinson’s treatment: symptoms improve in stem-cells trials
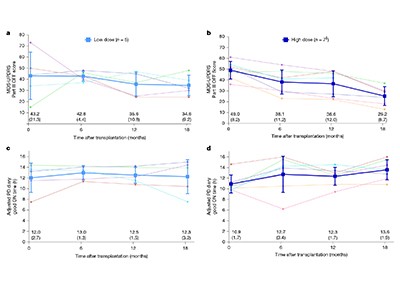
Tabar et al.2 describe the outcome of a phase I clinical trial in which 12 people in the United States and Canada were treated with dopaminergic progenitor cells derived from a human embryonic stem-cell line. In individuals who received a high dose of the therapy, scores used to assess of Parkinson’s disease symptoms were reduced by 50% at 18 months compared with the baseline measurements before transplantation. Furthermore, brain scans using a radioactive version of levodopa confirmed the survival of the transplanted cells and indicated an increase in dopamine production. There was no incidence of dyskinesia (involuntary movements), which had been a problem with fetal-tissue transplants.
The results are encouraging because they show that the use of allogeneic (non-self) transplants for the treatment of Parkinson’s disease is likely to be safe. To confirm their effectiveness, however, more research is needed. These two trials were small, open-label studies in which both the investigators and the participants were aware of who received what treatment. Because of this, there is a possibility that the results on efficacy were influenced by the placebo effect or investigator bias. Nevertheless, the fact that both independent trials proved to be safe, and hinted at possible efficacy, is an important step towards the establishment of this cell therapy for Parkinson’s disease in wider society. The next stages of clinical trials, phases II and III, are awaited to fully assess the efficacy of these interventions.
Competing Interests
H.O. is a co-founder of SanBio Co. Ltd. and K Pharma Inc., an unpaid board member (the president-elect) of the International Society for Stem Cell Research (ISSCR) and an unpaid board member (the president) of the Japanese Society for Regenerative Medicine (JSRM).