In 2021, cancer accounted for almost 15% of all deaths around the world, making it the second most common cause of human death after cardiovascular diseases. By 2040, an estimated 28 million new cases of cancer will be diagnosed every year globally, up from around 20 million in 2020. Advances in diagnosis and treatment must keep up with the growing burden of cancer, and clinical trials are an important part of such work.
Nature Index 2025 Cancer
“In a perfect world, every cancer patient should get to be part of a clinical trial if they want to be,” says Lola Rahib, an independent biomedical researcher and cancer patient advocate. This is, of course, not feasible, but it emphasizes the importance of decisions around which clinical trials to run and support, including which cancer types are prioritized and which patient demographics are studied.
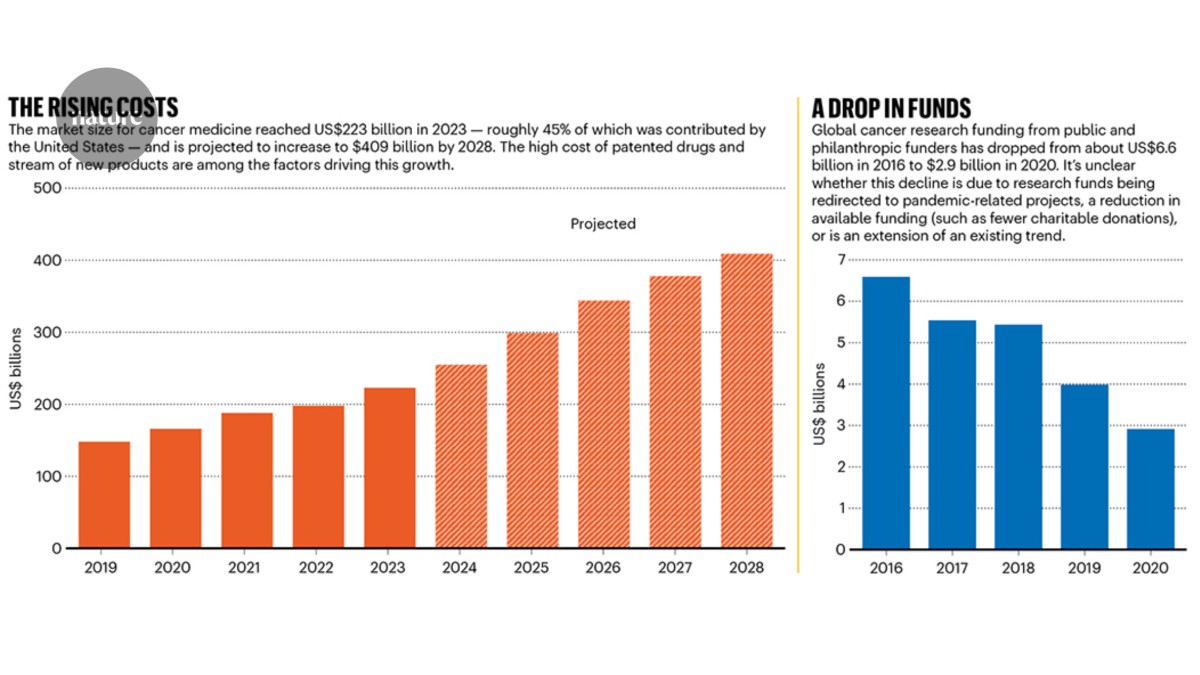
Choices about which therapeutics and technologies to invest in can also be hugely consequential for cancer patients, especially at a time when the costs of medications are skyrocketing. The market size for oncology drugs is expected to reach US$409 billion by 2028, up from $223 billion last year, according to a report1 by the IQVIA Institute for Human Data Science, an analytics and research company in Durham, North Carolina (see ‘The rising costs’). IQVIA attributes this increase to the large volume of patented medications on the market and the number of new cancer treatments that have been introduced in the last five years.
An agile presence
Smaller companies have solidified themselves as key players in cancer clinical trials (see ‘Emerging leaders’). IQVIA reports that emerging biopharmaceutical companies (classified as those with less than $500 million in annual sales and less than $200 million per year spent on R&D) were responsible for 60% of oncology trials in 2023, up from 33% in 2014. Large pharmaceutical companies (those with greater than $10 billion in annual sales) were responsible for 28% trial starts in 2023, down from 59% in 2014.
Many of these emerging companies are in China, which is quickly expanding its capacity to conduct cancer trials and research. A decade ago, Chinese companies were responsible for just 5% of new clinical trials, but by 2023, that figure had grown to 35%, the IQVIA report shows. Chinese companies now start more clinical trials than companies based in either the United States or European Union. This mirrors China’s trajectory in cancer research. In 2024, China overtook the United States as the leading country for high-quality cancer-research output in the Nature Index, with a Share of 2,614.52 — a 19% increase on the previous year. The United States, whose cancer-related Share was 2,481.71 in 2024, recorded a more modest increase in output of 5%.
The surge in oncology research from Chinese companies might take some time to affect the market. The IQVIA report highlights Chinese pharmaceutical company Jiangsu Hengrui, based in Lianyungang, as having a very strong pipeline of cancer therapies but relatively modest revenues. With 45 “unique assets” (drug and drug candidates) in various stages of development, Jiangsu Hengrui has a more packed pipeline than world-leading firms such as Roche, which has 37, and Pfizer, which has 22. Yet Jiangsu Hengrui’s cancer-drug revenues, at less than $800 million, are modest compared with many of its competitors, IQVIA reports. Rahib expects that Chinese pharmaceutical company revenues will increase rapidly in the coming years, as more of their therapeutics enter the market.