David Baker, Demis Hassabis and John Jumper (left to right) won the chemistry Nobel for developing computational tools that can predict and design protein structures.Credit: BBVA Foundation
For the first time — and probably not the last — a scientific breakthrough enabled by artificial intelligence (AI) has been recognized with a Nobel prize. The 2024 chemistry Nobel was awarded to John Jumper and Demis Hassabis at Google DeepMind in London, for developing a game-changing AI tool for predicting protein structures called AlphaFold, and David Baker, at the University of Washington in Seattle, for his work on computational protein design, which has been bolstered by Al in recent years.
“I hope when we look back on AlphaFold, it will be the first proof point of AI’s incredible potential to accelerate scientific discovery,” Hassabis said at a press briefing at DeepMind on 9 October. “It’s so unreal at this moment.”
What’s next for AlphaFold and the AI protein-folding revolution
The impact of AlphaFold, which was unveiled just a few years ago, has been nothing short of transformative. The tool has made protein structures — often, but not always, highly accurate ones — available to researchers at the touch of a button, and enabled experiments that were unimaginable a decade ago. “It’s a major revolution,” says Christine Orengo, a computational biologist at University College London, whose laboratory has used AlphaFold-predicted structures to uncover new proteins.
“It has long been a dream to learn to predict the three-dimensional structure of proteins from knowing their amino acid sequences … for several decades, this was considered impossible,” said Nobel committee chair Heiner Linke, who researches nanoscience at Lund University in Sweden, during the prize announcement. This year’s laureates “have cracked the code”, he added. The three winners share a prize pot of 11 million Swedish kronor (US$1 million).
Award-winning AI
DeepMind debuted AlphaFold in 2018, when it won a biennial protein-structure prediction contest called the Critical Assessment of Protein Structure Prediction (CASP). But it was the second iteration of the deep-learning neural network, revealed in late 2020, that really shook up the life sciences. Many of AlphaFold2’s predictions at CASP were so accurate as to be indistinguishable from experimentally solved protein structures.
Hassabis, DeepMind’s co-founder and chief executive, and Jumper, head of the AlphaFold team, led the development of AlphaFold2. To predict protein structures, the neural network incorporates data from libraries of hundreds of thousands of structures and millions of sequences from related proteins — which hold information about their shapes.
How to win a Nobel prize: what kind of scientist scoops medals?
In particular, AlphaFold’s success is due in no small part to the Protein Data Bank, a freely available repository of more than 200,000 protein structures determined using methods including X-ray crystollagraphy and cryo-electron microscopy. “It’s humbling every time we train [AlphaFold] on years of effort. Each data point is years of effort from someone,” Jumper said at the DeepMind press briefing.
In 2021, DeepMind made AlphaFold2’s underlying code freely available, along with the data needed to train the model. An AlphaFold database, created with the European Molecular Biology Laboratory’s European Bioinformatics Institute in Hinxton, UK, now holds the structures of almost all the proteins from every organism represented in genetic databases, some 214 million predictions in total. This year, the company unveiled a third version of AlphaFold, which can model other molecules that interact with proteins such as drugs.
The revolution that Jumper, Hassabis and their colleagues unleashed is still in its early days, and AlphaFold’s full impact on science might not be known for years. Already, the tool is helping scientists make new insights.
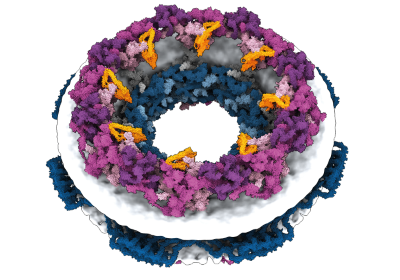
One pioneering team used the tool, along with experimental data, to map the nuclear pore complex, one of our cells’ largest machines that transports molecules into and out of the nucleus. Last year, two teams mined the entire AlphaFold database to uncover the darkest corners of the protein universe, identifying new families of proteins and folds and surprising connections in the machinery of life.
‘The entire protein universe’: AI predicts shape of nearly every known protein
Many researchers hope that AlphaFold and other AI tools it has inspired will transform medicine, but it is not yet clear how, or indeed whether, AlphaFold will streamline the costly and multi-step process of developing safe drugs. Scientists laying the groundwork for new vaccines are finding AlphaFold incredibly useful and, in some cases, game changing. But AlphaFold is a complement to experimental studies and other approaches to mapping and tweaking the structure of viral proteins for use in vaccines.
For most researchers, a predicted structure is the beginning of a study, not the end, says Jan Kosinski, a structural modeller at the European Molecular Biology Laboratory (EMBL) in Hamburg, Germany. “At the beginning, there was this fear that it will replace structural biology, people will lose jobs and so on. Actually, the complete opposite has happened,” he adds.
David Jones, a bioinformatician at University College London who collaborated with DeepMind on the first version of AlphaFold starting in 2016, says one of the tool’s biggest impacts has been a change in biologist’s mindset, “to say that computers are things that can produce useful hypotheses that can be tested in the lab”.
Creating new proteins
More than two decades before DeepMind started working on AlphaFold, computational biophysicist David Baker and his colleagues developed a software tool called Rosetta that modelled protein structures using physical principles. The tool compares small fragments of multiple existing protein structures and sequences to identify a protein sequence that can fold into a particular shape.
Initially, Rosetta was applied to predicting protein structures — it has been among the top entries at numerous CASPs, prior to AlphaFold’s dominance. But Baker soon realised that the model could be flipped around to design entirely new proteins.
AI tools are designing entirely new proteins that could transform medicine
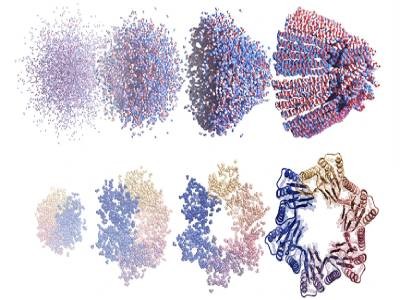
The tool had early success designing novel proteins, including new kinds of enzymes, proteins that can bind tightly to other molecules and self-assembling protein nanoparticles that resemble viruses (one of these served as a basis for an approved COVID-19 vaccine).
When AlphaFold2 was announced — but not yet released — Baker and his team, including computational chemist Minkyung Baek, now at Seoul National University in South Korea, set out to understand the software and apply some of its tricks to a previous AI-based version of Rosetta. The first version of the resulting RoseTTAFold network performed nearly as well as AlphaFold2. Since 2021, both networks have been continually improved by their developers and other scientists to tackle fresh challenges, such as predicting the structure of complexes of multiple different interacting proteins.
In recent years, Baker’s team have been especially prolific in applying machine learning to his lab’s raison d’etre: creating new proteins never seen before in nature. A tool recently developed by Baker’s team that melds RoseTTAFold with image-generating diffusion neural networks has led to a step-change in researchers’ capacity to design proteins.
Rapid progress
Such tools have been a massive accelerator and democratizer, says Sergey Ovchinnikov, an evolutionary biologist at the Massachusetts Institute of Technology in Cambridge, Massachusetts, who did his PhD in Baker’s lab. It used to take Rosetta weeks of running on hundreds of processors to come up with a protein design, a task that newer AI-based tools can accomplish in seconds. “Now everybody in the world can do protein design,” he says.
“I have been really deeply inspired by the others in the field and the people I’ve worked with,” said Baker, speaking by telephone at the Nobel prize announcement. “I stood on the shoulders of giants.”
Medicine Nobel awarded for gene-regulating ‘microRNAs’
Martin Steinegger, a computational biologist at Seoul National University in South Korea, likens the impact of AlphaFold, RoseTTAFold and other biological AI tools to that of the Apollo Moon missions by showing what engineering can achieve. “This is a similar moment for structure prediction and the structural biology field — just seeing what is possible,” he says.
Few were surprised with the Nobel committee’s decision. For Baker, “most people thought that it was ‘a when not if’ situation, the amount of work he’s done in that field,” says Jones. Jumper, aware that he and Hassabis were on many people’s short lists, said in the press briefing that he was unable to sleep the night before today’s announement.
For Jumper, the predicted structures that AlphaFold delivers create new opportunities for scientific discovery. Millions of scientists have already used the tools, and he hopes it won’t be long before one of them gets a call from Sweden. “The moment that I will be almost as excited as this will be the Nobel Prize that talks about the work done with AlphaFold,” he said.