General experimental procedures
Primer synthesis and DNA sequencing were performed by Genewiz. Purification of recombinant plasmids was performed using an EZNA Plasmid DNA Mini Kit from Omega Bio-Tek. Restriction enzymes were purchased from New England Biolabs and digestions were performed according to the manufacturer’s protocols. Gibson Master Mix was purchased from New England Biolabs and Gibson Assembly was performed according to the manufacturer’s protocol. Nickel nitriloacetic acid agarose (Ni-NTA) resin was purchased from Qiagen and Thermo Fisher Scientific. Benzylidenehydrazine was purchased from AstaTech. NADPH was purchased from Fisher Scientific. DBCO-acid was purchased from Conju-Probe. Diazoacetone was purchased from Enamine. Novex Tris-Glycine SDS–PAGE gels were purchased from Thermo Fisher Scientific. Protein concentrations were determined by measuring UV-absorption at 280 nm using a Thermo Scientific NanoDrop 2000. ExPASy ProtParam was used to calculate extinction coefficients. Optical densities of E. coli cultures were measured at 600 nm using a Beckman Coulter DU730 Life Sciences UV/Vis spectrophotometer. All water used was purified using a MilliQ water purification system. The remaining materials were purchased from Sigma Aldrich unless otherwise indicated. All experiments were performed in biological triplicates unless indicated and either individual data points with errors bars (± 1 s.d.) or representative results are shown. All starting materials used for chemical synthesis were either purchased from Sigma Aldrich or accessed synthetically as described below.
Sample preparation method 1
Ten microlitres of reaction mixture were diluted with 150 µl of LC–MS grade H2O, 20 µl of LC–MS grade acetonitrile (ACN), and 20 µl of LC–MS grade methanol and samples were filtered (0.2 µm, VWR, nylon).
Sample preparation method 2
Reaction mixtures were diluted with 70 µl of exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol), 15 µl of LC–MS grade methanol, and 15 µl of LC–MS grade ACN and filtered (3 kDa, Pall, Omega membrane)
Agilent 6530 qTOF spectrometer with Dual AJS source
Unless otherwise indicated, samples were analysed by LC–MS using an Agilent 1200 series LC system coupled to an Agilent 6530 qTOF spectrometer with Dual AJS source. Drying gas temperature was 300 °C, drying gas flow was 11 l min−1, nebulizer pressure was 45 psi, sheath gas temperature was 275 °C, sheath gas flow was 11 l min−1, capillary voltage was 3,500 V, nozzle voltage was 500 V, fragmentor voltage was 125 V, and skimmer voltage was 65 V. Mass spectra were acquired in positive ion mode. A mass window of 10 ppm was used for EICs.
Thermo Orbitrap IQ-X Tribrid
Unless otherwise indicated, samples were analysed using a Horizon Vanquish UHPLC coupled to a Thermo Orbitrap IQ-X Tribrid mass spectrometer. The LC column was a Kinetex C18 column (1.7 µm, 100 Å, 150 × 2.1 mm, Kinetex). Two microlitres of sample were injected. The flow rate was 0.4 ml min−1 using mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in ACN). The LC conditions were 0–2 min 10% B isocratic, 2–29 min 10–90% B, 29–32 min 90% B isocratic, 32–32 min 90–10% B, 32–35 min 10% B isocratic. The MS settings were: mass range 400–600 m/z, 120,000 resolution, the RF lens was 35%, the standard AGC target was used, and the auto maximum injection time mode was used. MS/MS spectra were acquired with a 1 m/z isolation window, 15,000 resolution, standard AGC target, auto maximum injection time mode and higher-energy collision-induced dissociation (HCD) fragmentation with 30% collision energy. Spectra were acquired in positive mode. A mass window of 5 ppm was used for EICs.
Comparative metabolomics using AcquireX and Compound Discoverer
Data-dependent MS/MS acquisition was performed via AcquireX (deep scan mode) from 4 replicate injections with media + DBCO-acid providing an exclusion list. Data-dependent MS/MS spectra were acquired with 2.0 × 104 intensity threshold, exclusion after 1 time, 2.5 s exclusion duration, a 3 ppm isolation window, and HCD, collision-induced dissociation (CID) and ultraviolet photodissociation (UVPD) fragmentation. HCD fragmentation was performed with a 1 m/z isolation window, stepped collision energies of 20, 35, 50, 75 and 100%, and 15,000 resolution. CID fragmentation was performed with a 1 m/z isolation window, 30% collision energy, 15,000 resolution, and 22 ms maximum injection time. UVPD fragmentation was performed with a 1.6 m/z isolation window, molecular weight-dependent UVPD activation time, 12% activation time, 15,000 resolution, and 22 ms maximum injection time.
Comparative metabolomics for t0 and t16 samples were performed using Compound Discoverer: The raw spectra of all samples were imported into Compound Discoverer 3.3 (Thermo Scientific), where peak alignment, compound detection, and compound grouping was performed. Compound detection was performed with a 3 ppm mass tolerance, a 10,000 minimum peak intensity, 1.5 chromatographic signal/noise threshold, and isotope grouping for Br and Cl ions. All ions were considered. Compound grouping was performed with a 3 ppm mass tolerance, a 0.2 min retention time tolerance, preferred ions of [M + H]+ and [M – H]– and area integration of the most common ion. Peak rating contributions were 3 for area contribution, 10 for coefficient of variation contribution, 5 for full width at half maximum (FWHM) to base contribution, 5 for jaggedness contribution, 5 for modality contribution, and 5 for zig-zag index contribution. Peak rating filters were 3 for peak rating threshold and 2 for number of files. Gap filling was performed with a mass tolerance of 5 ppm and a signal to noise threshold of 1.5. Systematic error removal using random forest (SERRF) QC correction was applied with a 50% minimum QC coverage, a 30% maximum QC area relative s.d., a 25% maximum corrected QC area relative s.d., 2 batches, and interpolated gap filling. Background compounds were marked with a maximum sample/blank ratio of 5, a maximum blank/sample ratio of 0 and the background compounds were then hidden. MS spectra were assigned as t16 or t0 and differential analysis was performed following spectra processing. Differential analysis was performed with area values transformed to log10. Peak rating contributions for differential analysis were 3 for area contribution, 10 for coefficient of variation contribution, 5 for FWHM to base contribution, 5 for jaggedness contribution, 5 for modality contribution, and 5 for zig-zag index contribution. Following statistical analysis, results were filtered to include only those with a calculated molecular weight and m/z greater than 333.
Reaction of azaserine with alkyne probes
One-hundred microlitre reaction mixtures containing 100 µM azaserine and 100 µM of the specified alkyne (10 mM stock solution in methanol) were prepared in water. The reaction mixtures were incubated for 16 h at room temperature. Samples were prepared using method 1. Detection of probe adducts was accomplished using the method described above for the Thermo Orbitrap IQ-X Tribrid mass spectrometer.
Optimization of the reaction with azaserine and DBCO-acid
Solvent screen
One-hundred microlitre reaction mixtures containing 100 µM azaserine and 100 µM DBCO-acid (10 mM stock solution in methanol) in H2O, 1:1 H2O:methanol, methanol, 1:1 H2O:ACN, or ACN were incubated overnight at room temperature. Samples were prepared using method 1. Detection of 3 was accomplished using the method described above for the Thermo Orbitrap IQ-X Tribrid mass spectrometer.
Reaction time screen
One-hundred microlitre reaction mixtures containing 100 µM azaserine and 500 µM DBCO-acid (10 mM stock solution in methanol) in H2O were incubated for the specified amount of time at room temperature. At the specified time point, samples were prepared using method 1. Detection of 3 was accomplished using the method described above for the Thermo Orbitrap IQ-X Tribrid mass spectrometer.
DBCO-acid equivalents
One-hundred microlitre reaction mixtures containing 100 µM azaserine and the specified equivalents of DBCO-acid (1 mM stock solution in methanol) in 10% methanol in H2O were incubated overnight at room temperature. Samples were prepared using method 1. Detection of 3 was accomplished using the method described above for the Thermo Orbitrap IQ-X Tribrid mass spectrometer.
DBCO-acid substrate scope
Two-hundred and fifty microlitre reaction mixtures containing 100 µM diazo substrate (azaserine stock solution: 25 mM in H2O; t-butyldiazoacetate, benzyldiazoacetate, ethyl diazoacetate stock solution: 25 mM in DMSO) and 500 µM DBCO-acid (50 mM stock solution in methanol) in H2O were incubated for 18 h at room temperature. Samples were prepared using method 1. Detection of DBCO-acid adducts was accomplished using the method described above for the Thermo Orbitrap IQ-X Tribrid mass spectrometer.
G. harbinensis
t
0 versus t
16 comparative metabolomics
G. harbinensis was grown on NZ amine plates (10 g l−1 glucose, 20 g l−1 soluble starch, 5 gl−1 yeast extract, 5 g l−1 NZ-amine, 1 g l−1 CaCO3, 15 g l−1 agar) at 30 °C for 7–10 days and was then used to inoculate 5 ml of NZ amine medium. The NZ amine culture was incubated for 7–10 days at 30 °C with shaking at 200 rpm. Four-hundred microlitres of the liquid culture were used to inoculate 20 ml of molasses production medium (10 g l−1 glucose, 5 g l−1 Bacto peptone, 20 g l−1 molasses, 1 g l−1 CaCO3) which was incubated at 30 °C with shaking at 200 rpm for 14 days. Two-hundred microlitres of culture were spun down for 5 min at maximum speed. One microlitre of 25 mM DBCO-acid in methanol was added to 49 µl of supernatant. The t0 control was obtained by dilution of 10 µl of the reaction mixture into 150 µl of LC–MS grade H2O + 20 µl of LC–MS grade ACN + 20 µl of LC–MS methanol, filtration (0.2 µm, VWR, nylon), and storage at –80 °C prior to analysis. The t16 sample was obtained from a 16 h incubation of the remaining reaction mixture at room temperature, followed by dilution of 10 µl of the reaction mixture into 150 µl of LC–MS grade H2O + 20 µl of LC–MS grade ACN + 20 µl of LC–MS grade methanol, and filtration (0.2 µm, VWR, nylon). Comparative metabolomics were performed using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above.
Compilation of diazo biosynthetic gene clusters
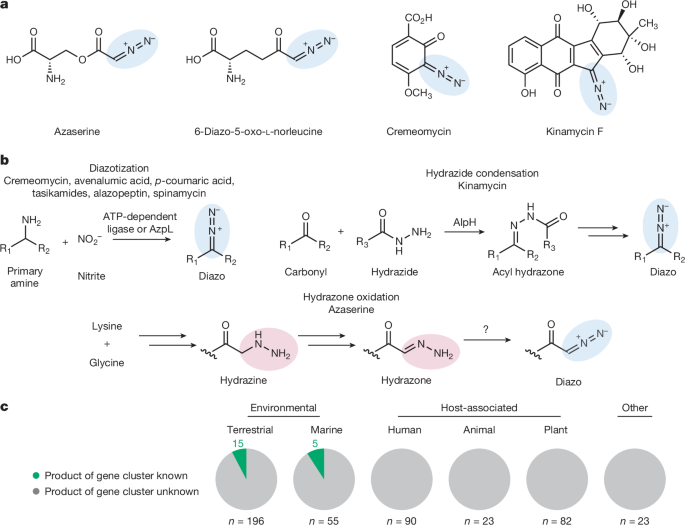
To compare the number of experimentally validated versus bioinformatically predicted biosynthetic gene clusters involved in diazo formation, we first performed a literature search to identify all experimentally verified diazo biosynthetic gene clusters5,12,14,15,16,17,18,20,21,22,38,55,56,57,58,59,60,61,62,63 (20 clusters identified; see Source Data for Fig. 1). We then performed a literature search to compile prior genome mining efforts to identify biosynthetic gene clusters potentially involved in diazo formation. Janso et al.55 identified homologues of putative diazo-forming enzymes by querying lom29, lom32, lom33, lom34 and lom35 against sequenced bacterial genomes (note: it is unclear whether gene or protein sequences were used for this search). Accession numbers and sequences for all protein sequences used as queries are provided in Supplementary Table 7. Protein sequences or accession numbers were provided if gene accession numbers could not be identified. Waldman performed a gene cluster architecture search using creD, creE and creM as queries against the GenBank database using the Integrated Microbial Genomes-Joint Genome Institute (IMG/JGI) MultiGeneBlast program56. Kawai et al.14 identified clusters containing azpL homologues by querying azpL against an unspecified genome database. Kawai et al.16,18 identified homologues of the ava gene cluster using BiG-SCAPE. Van Cura et al.40,41 identified homologues of the aza gene cluster by using the EFI-GNT to query AzaDHILNP against the Uniprot database20. Shikai et al.22 identified homologues of the azs gene cluster using antiSMASH and BiG-SCAPE. Compiling predicted gene clusters from these studies and dereplicating based on strain name and query sequence yielded 382 bioinformatically predicted diazo biosynthetic gene clusters. Compilation of these results with the 87 biosynthetic gene clusters identified through our genome mining (see Source Data for Fig. 1) resulted in Fig. 1c. Because prior studies were performed using earlier versions of databases, the actual number of predicted diazo biosynthetic gene clusters is likely to be greater than 382.
Genome mining for biosynthetic gene clusters involved in hydrazone N-oxidation
In short, homologues of the hydrazine-forming enzyme AzaE were identified via a BLAST search of the NCBI reference protein database (refseq_protein). The genes neighbouring these homologues were retrieved using prettyClusters39 to identify putative biosynthetic gene clusters. Manual filtering was performed to select only gene clusters containing Pfams consistent with putative hydrazone biosynthetic enzymes and an additional oxidoreductase.
To begin this analysis, the protein sequence of AzaE (WP_091038156.1) was queried against the NCBI nucleotide collection database (November 2023) and the non-redundant protein sequence database in tblastn and blastp searches, respectively. Blastp results with an E value <10−98 that were non-redundant were added to the tblastn search results. GenBank files were downloaded using the accession number for each result. The files were prepared according to the prettyClusters39 workflow, detailed below. The GenBank documents were annotated with Prokka64. Amino acid sequences were extracted and added to a local database. AzaE was queried against this database in a blastp search and homologues with an E value <10−31 were taken forward. Gene and neighbour metadata and neighbour sequences were generated with the gbToIMG function using default values except NeighborNumber was set to 15. InterProScan was used to generate additional metadata for all genes and neighbours. These data were merged with the previous metadata using the incorpIprScan function. The prepNeighbors function was run with NeighborNumber = 10 and trimShortClusters = false. AzaE homologues were dereplicated using the Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST)40,41 tool and the repNodeTrim function. Manual curation identified gene neighbourhoods containing homologues of hydrazone-forming enzymes (neighbourhoods contained at least one representative each of Pfam02770, Pfam13434, Pfam01266, Pfam00501, Pfam13302, and Pfam03417; Pfam00550 was not considered as acyl carrier proteins are commonly not annotated). Additional curation identified gene neighbourhoods containing an additional oxidoreductase as indicated by Pfam annotations. The analyzeNeighbors function was run on the clusters containing the hydrazone biosynthetic enzymes with neighborThreshold = 2.5 and tgCutoff = 56. Results were visualized with Cytoscape65. This analysis identified 129 gene clusters. Of these, 2 were related to s56-p1 biosynthesis and 14 were related to triacsin biosynthesis, yielding 113 putative diazo biosynthetic gene clusters. Of these, 26 were previously identified as putative azaserine biosynthetic gene clusters and dereplication of these clusters yielded 87 unique clusters. Combining these results with the 382 biosynthetic gene clusters identified in previous studies resulted in Fig. 1c (see Source Data for Fig. 1). While the NCBI reference protein database contains proteins from a variety of source,s including plants, animals and fungi, hits were observed only from bacterial sources.
Diazo-containing natural product discovery from N. ninae
N. ninae was grown on GYM agar (4 g l−1 glucose, 4 g l−1 yeast extract, 10 g l−1 malt extract, 2 g l−1 CaCO3, 20 g l−1 agar) plates for 7–10 days at 30 °C. Colonies were used to inoculate 5 ml of GYM medium (4 g l−1 glucose, 4 g l−1 yeast extract, 10 g l−1 malt extract), and the culture was incubated for 7–10 days at 30 °C with shaking at 200 rpm. One-hundred microlitres of the culture were used to inoculate 5 ml of GYM medium and this was incubated for 7–10 days at 30 °C with shaking at 200 rpm. Two-hundred microlitres of spent culture medium were spun down at maximum speed for 5 min. One microlitre of 25 mM DBCO-acid in methanol was added to 49 µl of spent culture medium and incubated for 16 h at room temperature. Samples were prepared using method 1. Samples were analysed using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z. Comparative metabolomics for t0 and t16 samples were performed using Compound Discoverer as described in the general methods above.
Detection of 2 by LC–MS
Compound 2 was detected using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z.
Detection of 4 and 5 from N. tenerifensis
N. tenerifensis was grown on GYM agar plates for 7–10 days at 30 °C. Colonies were used to inoculate 5 ml of GYM medium and the culture was incubated for 7–10 days at 30 °C with shaking at 200 rpm. One-hundred microlitres of the culture were used to inoculate 5 ml of molasses production medium, and this culture was incubated for 7–10 days at 30 °C with shaking at 200 rpm. Two-hundred microlitres of spent culture medium were spun down at maximum speed for 5 min. One microlitre of 25 mM DBCO-acid in methanol was added to 49 µl of spent culture medium and incubated overnight at room temperature. Samples were prepared using method 1. Detection of 4 and 5 was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z. MS/MS was accomplished using HCD fragmentation with 30% normalized collision energy or CID fragmentation with 30% normalized collision energy, 10 ms activation time, and 0.25 activation Q for 5 and 4, respectively.
Cloning dob-pDualP
Construction of the dob-pDualP vector was performed as previously reported for aza-pDualP, with minor modifications20. Cloning of dob-pDualP was performed by Terra Bioforge. N. ninae cells were lysed using proprietary methods to maintain genomic DNA integrity. Genomic DNA > 50 kb was isolated using proprietary gel free methods. The dob gene cluster was excised from N. ninae genomic DNA using Cas9 with guide RNAs targeting upstream and downstream of the gene cluster. A Streptomyces compatible dual-inducible vector (pDualP) containing the Potr inducible expression system66 (oxytetracycline) and PnitA67,68 inducible promoter (ε-caprolactam) flanking the insertion site was amplified using PCR with primers designed to create ~40 bp of overlap specific to the dob gene cluster. Modified Gibson Assembly of the dob gene cluster and PCR amplified linear pDualP was performed using a proprietary reaction mix to produce the dob-pDualP expression vector. The dob-pDualP vector was transformed into E. coli BacOpt2.0 (E. coli DH10b derivative) and clones were verified by whole plasmid sequencing.
Conjugation of dob-pDualP into S. coelicolor M1152
S. coelicolor M1152 was grown on MSF agar (20 g l–1 mannitol, 20 g l–1 soy flour, 20 g l–1 agar) for 7 days at 30 °C. A single colony was used to inoculate 5 ml of YEME medium (3 g l−1 yeast extract, 3 g l−1 malt extract, 5 g l−1 peptone, 10 g l−1 glucose, 340 g l−1 sucrose), which were incubated at 30 °C with shaking at 200 rpm for 7 days. Five millilitres of LB + apramycin (50 µg ml−1) + kanamycin (50 µg ml−1) + chloramphenicol (20 µg ml−1) medium were inoculated with E. coli ET12567/pUZ8002 dob-pDualP and grown at 37 °C with shaking at 200 rpm for 2 days. The culture was spun down at 3,000 rpm for 10 min. Five millilitres of LB medium were added and the pellet was resuspended. The resuspension was spun down at 3,000 rpm for 10 min and the supernatant was discarded. This wash step was then repeated before the pellet was resuspended in 100 µl of LB medium. The S. coelicolor culture was spun down at 4,000 rpm for 10 min on the same day. Two millilitres of sucrose (10% w/v in water) were added, the pellet was resuspended, and the resuspension was spun down at 4,000 rpm for 10 min. This was repeated once before the pellet was resuspended in 100 µl of LB. One microlitre of S. coelicolor suspension were combined with 100 µl of E. coli suspension and mixed. This was plated on MSF + 10 mM MgCl2 agar and grown at 30 °C. After 18 h, the plate was flooded with 1 ml of sterile water containing 1 mg apramycin and 0.5 mg nalidixic acid before being incubated at 30 °C. Ex-conjugants were grown on MSF + apramycin (50 µg ml−1) + nalidixic acid (30 µg ml−1) agar and incorporation of dob-pDualP was confirmed by genome sequencing.
Detection of 4 and 5 from S. coelicolor dob-pDualP
S. coelicolor dob-pDualP was grown on MSF + nalidixic acid (30 µg ml−1) + apramycin (50 µg ml−1) agar plates at 30 °C for 5–7 days and then used to inoculate 5 ml of YEME medium. Wild-type S. coelicolor was grown on MSF agar plates at 30 °C for 5–7 days and used to inoculate 5 ml of YEME medium. The YEME cultures were incubated at 30 °C with shaking at 200 rpm for 7 days. One-hundred microlitres of the YEME cultures were used to inoculate 5 ml of molasses production medium which was incubated at 30 °C with shaking at 200 rpm for 14 days. ε-caprolactam and oxytetracycline were added to the cultures for final concentrations of 0.1% w/v and 2.5 mM, respectively. After incubation of the induced cultures at 30 °C with shaking at 200 rpm for 7 days, 200 µl of spent culture medium were derivatized and analysed as described above with minor modifications. The MS settings were: mass range 400–480 m/z. MS/MS spectra were acquired with a 5 ppm isolation window, 15,000 resolution, 1,000% AGC target, dynamic maximum injection time, and HCD fragmentation with 30% normalized collision energy or CID fragmentation with 30% collision energy, 10 ms activation time, and 0.25 activation Q for 5 and 4, respectively.
Estimation of the concentration of 5 in derivatized culture supernatants
Compound 5 was diluted to 50 µM, 5 µM and 500 nM, in GYM medium or 10 µM, 1 µM, and 100 nM in molasses or ISP4 medium. Samples were prepared in biological triplicates. N. ninae, N. tenerifensis, and S. coelicolor dob-pDualP were cultured and their supernatants derivatized with DBCO-acid as described above. LC–MS samples for the derivatized supernatants and standard curves were prepared using method 1 and analysed using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above for the detection of 5 from N. tenerifensis. The EIC of 5 was extracted ± 5 ppm and the combined peak area of the regioisomeric peaks was plotted in GraphPad. A simple linear regression was performed using the GraphPad software. Each replicate y value was considered as an individual point and the range of the regression line was automatically calculated. The equation of the resulting regression line was then used to approximate the concentration of 5 in the derivatized supernatants.
Cloning dob-pDualP Δdob3
The deletion of Dob3 was performed by Terra Bioforge. In brief, dob-pDualP was digested in vitro with CRISPR nuclease complexed with two guide RNAs targeting both the 5′ and 3′ ends of the FDO gene. The digested DNA was then repaired in an isothermal assembly reaction with the AmpR cassette from pUC19, which was amplified by PCR using the primers 5′-gaaatggagggatagccgtgtaagtttaaacggcacttttcgggg-3′ and 5′-aaattggacagcagccgcttgtttaaacgttaccaatgcttaatcagtgagg-3′. The assembly reaction to remove the FDO gene and replace it with the AmpR cassette was then transformed via electroporation into E. coli DH10B. The transformation culture was then plated on LB agar containing 25 µg ml−1 apramycin and 100 µg ml−1 ampicillin. Transformant colonies were picked, and plasmid DNA was extracted. Whole plasmid sequencing was performed by Plasmidsaurus using Oxford Nanopore Technology with custom analysis and annotation.
Conjugation of dob-pDualP Δdob3 into S. coelicolor M1152
Five millilitres of LB + apramycin (25 µg ml−1) + chloramphenicol (10 µg ml−1) + kanamycin (25 µg ml−1) medium were inoculated with E. coli ET12567/pUZ8002 dob-pDualP Δdob3 and grown at 37 °C with shaking at 200 rpm overnight. After the culture reached OD600 > 0.4, it was spun down at 3,000 rpm for 10 min and the supernatant was discarded. The pellet was resuspended in 5 ml of LB medium. The resuspension was spun down at 3,000 rpm for 10 min and the supernatant was discarded. This wash step was repeated once before the pellet was resuspended in 500 µl of LB medium. Ten microlitres of S. coelicolor M1152 spore stock were added to 500 µl of 2× YT medium (16 g l–1 casein digest peptone, 10 g l–1 yeast extract, 5 g l–1 NaCl) and incubated for 10 min at 50 °C. Samples were then cooled to room temperature. Five-hundred microlitres of E. coli ET12567/pUZ8002 dob-pDualP ΔDob3 in LB medium were added to 500 µl of S. coelicolor in 2× YT medium. Samples were spun down at 3,000 rpm for 10 min and 850 µl of supernatant were removed. The pellet was resuspended in the remaining supernatant and plated on MSF + 50 mM MgCl2 agar. The plate was then incubated at 30 °C for 24 h before it was flooded with 1 ml of sterile water containing 1 mg of apramycin and 0.5 mg of nalidixic acid. Ex-conjugants were grown on MSF + apramycin + nalidixic acid agar and incorporation of dob-pDualP ΔDob3 was confirmed by genome sequencing.
Genome sequencing verification of S. coelicolor dob-pDualP Δdob3
S. coelicolor dob-pDualP and S. coelicolor dob-pDualP Δdob3 were grown on MSF + apramycin (50 µg ml−1) agar for 7 days at 30 °C. Single colonies were used to inoculate 5 ml of YEME medium (3 g l−1 yeast extract, 3 g l−1 malt extract, 5 g l−1 peptone, 10 g l−1 glucose, 340 g l−1 sucrose) which were incubated for 7 days at 30 °C with shaking at 200 rpm. Genomic DNA was extracted using the Lucigen MasterPure Gram Positive gDNA Purification Kit. Samples were incubated with Ready-Lyse lysozyme for 36 h and eluted with 10 µl of H2O. Genomic DNA sequencing was performed by Plasmidsaurus using Oxford Nanopore Technology with custom analysis and annotation.
Comparison of S. coelicolor dob-pDualP versus S. coelicolor dob-pDualP Δdob3 spent media
Comparison of S. coelicolor dob-pDualP and S. coelicolor dob-pDualP Δdob3 spent media was performed using the method described above for the detection of 4 and 5 from S. coelicolor dob-pDualP with the following modification: 100 µl of YEME starter culture were used to inoculate 5 ml of ISP4 medium (10 g l−1 soluble starch, 1 g l−1 MgSO4•7H2O, 1 g l−1 NaCl, 2 g l−1 (NH4)2SO4, 2 g l−1 CaCO3 and 1 ml of trace salts solution (stock solution: 1 g l−1 FeSO4•7H2O, 1 g l−1 MnCl2•4H2O, 1 g l−1 ZnSO4•7H2O)).
Isolation of N. ninae genomic DNA
N. ninae was grown on GYM agar plates for 7–10 days at 30 °C. Single colonies were used to inoculate 5 ml of GYM medium and the culture was incubated for 7–10 days at 30 °C with shaking at 200 rpm. Genomic DNA was extracted using the Lucigen MasterPure Gram Positive gDNA Purification Kit with a 16 h lysozyme incubation. Samples were eluted with 10 µl of H2O.
Cloning DobA, DobB, DobQ, DobC, DobG, DobM, Dob2 and Dob3
Genes encoding each protein were amplified by PCR using the appropriate forward and reverse primers listed in Supplementary Table 1. PCR products were isolated using Zymoclean Gel DNA Recovery Kit and DNA Clean & Concentrator. PCR products were ligated into a NdeI/HindIII-digested pET28a plasmid using Gibson Assembly. Plasmids were transformed into E. coli Top10 and the sequence was confirmed by Sanger sequencing. Plasmids containing the dobA, dobB, dobC, dobG, dobM and dob3 inserts were transformed into E. coli BL21(DE3) for protein expression. Plasmids carrying the dobQ (carrier protein) and dob2 (PKS) inserts were transformed into E. coli BAP1 (ref. 69) for expression of phosphopantetheinylated holoenzymes.
Site-directed mutagenesis
Dob3 point mutants were constructed by adapting the Agilent QuikChange protocol70. PCR reactions contained 18 µl of H2O, 1 µl of pET28a-Dob3 (86.1 ng µl−1), 2 µl of DMSO, 25 µl of Q5 High-Fidelity 2× Master Mix and 1 µl of appropriate primers (10 µM stock) listed in Supplementary Table 1. Two microlitres of DpnI were used to digest source plasmids following amplification. Plasmids were transformed into E. coli Top10 and the sequence was confirmed by Sanger sequencing. Plasmids containing the mutated inserts were transformed into E. coli BL21(DE3) for protein expression.
Expression and purification of DobA, DobB, DobC, DobG, DobM, DobQ, Dob2, Dob3 and Dob3 variants
A single colony was used to inoculate 30 ml of LB medium + kanamycin (50 µg ml−1) which were incubated overnight at 37 °C with shaking at 200 rpm. A 20 ml aliquot was used to inoculate 1 l of LB medium + kanamycin (50 µg ml−1) culture which was incubated at 37 °C with shaking at 180 rpm until an OD600 of at least 0.6 was reached. Cultures were cold-shocked on ice for 30 min before induction with 50 µg ml−1 isopropyl β-d-1-thiogalactopyranoside (IPTG). For DobG and DobM, 75 mg l−1 riboflavin were added after induction. Cultures were incubated at 18 °C with shaking at 180 rpm overnight. After 16 h, cells were centrifuged at 4,000 rpm at 4 °C for 20 min. Pellets were resuspended in 30 ml of 20 mM HEPES pH 8.0, 10 mM MgCl2, and 500 mM NaCl. For DobG and DobM, 1 mM FAD was added to the lysis buffer. Cells were lysed using a cell disruptor (Avestin EmulsiFlex-C3) and lysates were spun down at 15,000g for 40 min at 4 °C. Spent culture medium was combined with 3 ml of Ni-NTA resin and incubated with gentle rotation at 4 °C for 30 min. The resin was washed with buffer containing 25 mM imidazole before eluting proteins in buffer containing 200 mM imidazole. Eluted proteins were concentrated to <2 ml using a MilliporeSigma Amicon Ultra-15 Centrifugal Filter Unit with an appropriate molecular weight cutoff. DobB, DobC, DobG, DobM, DobQ, Dob2, Dob3 and Dob3 mutants were desalted using buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) and Cytiva PD-10 columns pre-packed with Sephadex G-25 resin. DobA was desalted and further purified on a Bio-Rad BioLogic DuoFlow FPLC system with a Superdex 75 column using 20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, and 10% glycerol as the mobile phase. Desalted proteins were concentrated using a MilliporeSigma Amicon Ultra-15 Centrifugal Filter Unit with an appropriate molecular weight cutoff before storing concentrated proteins at –80 °C. To determine the molecular weight of Dob3, Bio-Rad Gel Filtration (1511901, batch 64656562) standards were analysed on a Bio-Rad BioLogic DuoFlow FPLC system with a Superdex 200 column using 20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, and 10% glycerol as the mobile phase. Dob3 was analysed using the same system with a Superdex 200 column using 20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, and 10% glycerol as the mobile phase to determine its molecular weight.
In vitro activity assay of DobG
The in vitro activity of DobG was investigated as previously described for the homologue AzaG20, with the modification that supernatants were additionally passed through a 0.2 µM filter (VWR, 13 mm, Nylon, 0.2 µm) prior to LC–MS analysis. MS/MS spectra were collected with 10 V collision energy.
In vivo activity assay of DobE
Analysis of in vivo activity of DobE was conducted as previously described for AzaE20.
In vitro activity assay of DobB
The in vitro activity of DobB was investigated as previously described for AzaB20 with the following modifications: After the reaction, samples were diluted 95:5 H2O:ACN and filtered (3 kDa, Amicon, cellulose).
Ppant ejection assay of DobA, DobB, DobC, DobM and DobQ
The Ppant ejection assay of DobA, DobB, DobC, DobM, and DobQ was conducted as previously described for their homologues AzaB, C, M, and Q20, with the modification that 40 µM DobA (AzaA homologue) was also added to the reaction mixtures.
In vitro activity assay of Dob3
Fifty-microlitre reaction mixtures containing 100 µM phenazine method sulfate (PMS), 20 µM Dob3, 1 mM 6-OMe (50 mM stock solution in methanol), 2 mM NADPH, 0.1 mg PLE, and 500 µM DBCO-acid (25 mM stock solution in methanol) in exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) were incubated at room temperature overnight. Samples were prepared using method 2. Detection of 4 and 5 was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z. MS/MS spectra of 5 were obtained using HCD fragmentation with 30% normalized collision energy. MS/MS spectra of 4 were obtained using CID fragmentation with 30% collision energy, 10 ms activation time and 0.25 activation Q.
DobA, DobB, DobC, DobM, DobQ, Dob2 and Dob3 cascade reaction
Fifty-microlitre reaction mixtures containing 1 mM 10, 20 µM DobB, 200 µM succinyl-CoA, 50 µM DobQ, 20 µM DobC, 5 mM ATP, 20 µM DobM, 200 µM FAD, 40 µM DobA, 10 µM Dob2, 200 µM malonyl-CoA, 20 µM Dob3, 100 µM PMS, 2 mM NADPH, and 500 µM DBCO-acid (25 mM stock solution in methanol) in exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) were incubated aerobically at room temperature for 16 h. Samples were prepared using method 2. Detection of 4 and 5 was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z. MS/MS spectra of 5 were obtained using HCD fragmentation with 30% normalized collision energy. MS/MS spectra of 4 were obtained using CID fragmentation with 30% collision energy, 10 ms activation time and 0.25 activation Q.
Optimization of Dob3 redox system
Fifty-microlitre reaction mixtures containing 1 mM 6–OMe, 20 µM Dob3, 500 µM DBCO-acid, and a redox system (2 mM NADPH + 100 µM PMS, 1 mM ascorbate, 1 mM dithiothreitol, 100 µg ml−1 spinach ferredoxin + 100 mU ml−1 spinach ferredoxin reductase + 1 mM NADPH, or no redox system) in exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) were incubated aerobically at room temperature overnight. Samples were prepared using method 2. Detection of 4-OMe was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z.
Optimization of Dob3 electron mediator
Fifty-microlitre reaction mixtures containing 1 mM 6-OMe, 2 mM NADPH, 20 µM Dob3, 500 µM DBCO-acid, and 100 µM electron mediator (phenazine methosulfate, phenazine ethosulfate, or methyl viologen) were incubated aerobically at room temperature overnight. Samples were prepared using method 2. Detection of 4-OMe was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z.
ICP-MS analysis of Dob3 expressed in LB
Sixty microlitres of trace-metal free nitric acid was added to 200 µl of 1 mg ml−1 Dob3 in water. This mixture was incubated for 3 h at 60 °C before precipitates were removed by centrifugation. Two-hundred microlitres of supernatant was diluted to a total volume of 3 ml with molecular biology grade water. Samples were analysed on an Agilent 7900 Inductive Coupled Mass Spectrometer.
ICP-MS analysis of Dob3 expressed in M9 with metal supplementation
A 105 µl volume of trace-metal free nitric acid were added to 350 µl of 1 mg ml−1 Dob3 in water. This mixture was incubated for 3 h at 60 °C before precipitates were removed by centrifugation. Four-hundred microlitres of supernatant were diluted to a total volume of 5 ml with molecular biology grade water. ICP-MS was performed at the Northwestern University Quantitative Bulk-Element Information Core on a computer-controlled (QTEGRA software) Thermo iCapQ ICP-MS (Thermo Fisher Scientific) operating in KED mode and equipped with a ESI SC-2DX PrepFAST autosampler. Nickel skimmer and sample cones were used from Thermo Scientific (part numbers 1311870 and 3600812). Internal standard was added inline using the prepFAST system and consisted of 1 ng ml−1 of a mixed element solution containing Bi, In, 6Li, Sc, Tb and Y (IV-ICP-MS-71D from Inorganic Ventures). Each sample was acquired using 1 survey run (10 sweeps) and 3 main (peak jumping) runs (40 sweeps). The isotopes selected for analysis were 55Mn, 56,67Fe, 59Co, 60,62Ni, 63,65Cu, 64,66,68Zn, 95Mo, and 45Sc, 89Y and 115In (chosen as internal standards for data interpolation and machine stability). Instrument performance was optimized daily through autotuning followed by verification via a performance report (passing manufacturer specifications).
Substrate scope of Dob3
Fifty-microlitre reaction mixtures containing 1 mM of the specified hydrazone substrate, 2 mM NADPH, 100 µM PMS, 20 µM Dob3, and 500 µM DBCO-acid (25 mM stock solution in methanol) in exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) were incubated overnight. Samples were prepared using method 2. Detection of corresponding pyrazoles was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above. HCD fragmentation with 30% normalized collision was used. Spectra were acquired in positive ion mode. A mass window of 5 ppm was used for EICs.
One-pot chemoenzymatic production of diazoacetone and diazomethylbenzene
Fifty-microlitre reaction mixtures containing 100 µM PMS, 20 µM Dob3 and 1 mM aldehyde (methylglyoxal or benzylidenehydrazine, 50 mM stock solutions in exchange buffer or methanol, respectively), 2 mM NADPH, 2 mM hydrazine hydrate, and 500 µM DBCO-acid (25 mM stock solution in methanol) in exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl and 10% glycerol) were incubated overnight. Samples were prepared using method 2. Detection of 5 and 20 was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z.
Anaerobic and aerobic hydrazone incubation
Fifty-microlitre reaction mixtures containing 500 µM benzylidenehydrazine (25 mM stock solution in methanol), 100 µM PMS, 2 mM NADPH, and 500 µM DBCO-acid (25 mM stock solution in methanol) in exchange buffer (20 mM HEPES pH 8.0, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) were incubated for 22 h at room temperature either aerobically or anaerobically. Samples were quenched aerobically or anaerobically through addition of 70 µl of buffer, 15 µl of LC–MS grade ACN, and 15 µl of LC–MS grade methanol and then filtered (0.2 µm, VWR, nylon). Anaerobic samples were moved to the autosampler approximately 5 min prior to injection. Detection of 20 was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z.
pH screen of hydrazone autoxidation
Fifty-microlitre reaction mixtures containing 500 µM benzylidenehydrazine (25 mM stock solution in methanol), 100 µM PMS, 2 mM NADPH, and 500 µM DBCO-acid (25 mM stock solution in methanol) in exchange buffer at the specified pH values (20 mM HEPES, 10 mM MgCl2, 50 mM NaCl, 10% glycerol) were incubated for 22 h at room temperature either aerobically or anaerobically. Samples were prepared using method 2. Detection of 20 was accomplished using a Thermo Orbitrap IQ-X Tribrid mass spectrometer as described above with minor modifications. The MS settings were: mass range 70–700 m/z.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.