Mouse strains and genotyping
All animal experiments were performed according to institutional and national guidelines approved by the Toronto Centre for Phenogenomics (TCP) and Cincinnati Children’s Hospital Medical Center. Mice were housed under controlled conditions (specific-pathogen free, 12 h:12 h light:dark cycle, 19–22 °C, 45–65% humidity) with ad libitum access to food and water. We added environmental enrichment in the form of plastic tunnels and nesting material to all the cages. Clinical symptoms define the welfare of animals. That includes visible masses, any degree of reduced mobility/distress, weight loss or evidence of haemorrhage. Humane intervention points are set based on institutional standard operating procedure (SOP) of Humane Intervention Point Guidelines and Cancer Models–Humane Intervention Point Guidelines. In brief, tumour size exceeding 1,700 mm3 in adult mice is considered the endpoint for cancer models. No mouse exceeded the humane endpoints stipulated in our animal SOPs. Both male and female mice were used in the study.
α-cre mice (P. Gruss, age E14–P400), B6.129P2(Cg)-Braftm1Mmcm/J (Jackson Laboratory, strain 017837, common name: BRafCA, age P0–P42), B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, also known as Ai14(tdTomato) (Jackson Laboratory, strain 007914, age P0–P91), B6.129-Gt (ROSA)26Sortm1(cre/ERT2)Tyj/J (Jackson Laboratory, strain 008463, age P0–P91), B6.129S4-Krastm4Tyj/J (Jackson Laboratory, strain 008179, age P0–P91), Cdk1f/f (D. Santamaria and M. Barbacid, age P0–P400), Cdk2f/f (D. Santamaria and M. Barbacid, age P0–P400), p107−/− mice (M. Rudnicki, age P0–P400), p27CK−/CK− mice (A. Besson and J. Roberts, age P0–P400), p27T187A (A. Besson and J. Roberts, age P0–P400), p53f/f mice (A. Berns, age E18–P56), Rbf/f mice (A. Berns, age E14–P400), Skp2−/− mice (K. Nakayama, age P0–P400), SPC-rtTA (Whitsett, age E18–P60), TetO-Cre (Whitsettage age E18–P60) and Z/Red (Jackson Laboratory, strain 005438, age E16) mice were maintained on a mixed background. Different genotypes were compared within the same litter and at least four to six litters. We have not noted any phenotypic differences in separate litters. Genotyping was performed as before and per The Jackson Laboratory guidelines using established primers (Supplementary Table 6).
α-cre retinoblastoma model
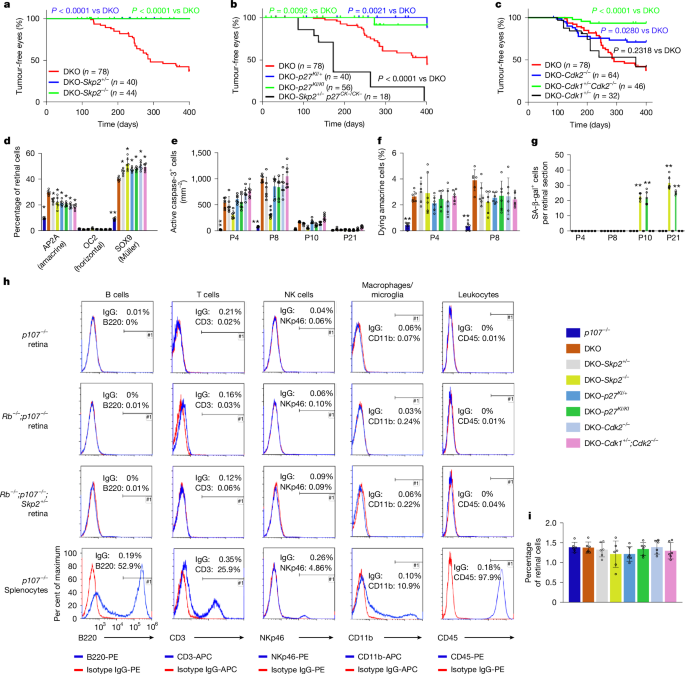
α-cre mice62 were mated with Rbf/f mice63,64 and p107−/− mice65 to generate α-cre; Rbf/f;p107−/− (Rb/p107 DKO) mice, an established retinoblastoma mouse model16. DKO mice were further mated with Skp2−/− (ref. 66), Cdk1f/f (ref. 32), Cdk2f/f (ref. 67), p27CK− (ref. 68) and p27T187A (p27KI)69 mice to elucidate the mechanism of tumorigenesis. Genotypes were determined by PCR using established primers (Supplementary Table 6). p107−/− littermates were used as controls.
Doxycycline-induce Rb and p53 double-knockout SCLC model
SPC-rtTA transgenic mice70 and tet-OCre transgenic mice46,47,71 were mated to Rbf/f mice63,64 and p53f/f mice72, to generate SPC-rtTA;tet-OCre;Rbf/f;p53f/f mice, an established SCLC animal model47. Genotypes were determined by PCR analysis using established primers46,73 (Supplementary Table 6). Gestational age was assigned by vaginal plug date designated embryonic day (E) 0.5. A 50× doxycycline stock solution (50 mg ml−1 in 50% ethanol) was freshly prepared prior to each administration of drug and diluted in water or in PBS for injection. Dams were treated with 125 μg doxycycline (Sigma, D9891) in 0.5 ml PBS by intraperitoneal injection on E0.5–E1.5 and administered doxycycline in the drinking water at a final concentration of 1.0 mg ml−1. Doxycycline water was replaced three times per week because of the light sensitivity of doxycycline.
Tamoxifen-induced Rb and p53 double-knockout SCLC model
Rosa-creERT2 and Ai14 (tdTomato) transgenic mice were mated to Rbf/f (refs. 63,64) and p53f/f mice72 to generate creERT2;tdTomato;Rbf/f;p53f/f mice, an established SCLC animal model47. Genotypes were determined by PCR analysis using established primers46,73 (Supplementary Table 6). creERT2;tdTomato littermate mice were used as control. Both creERT2; tdTomato;Rbf/f;p53f/f mice and control mice were treated with 100 mg kg−1 body weight Tamoxifen (Sigma, T5468) in 0.45 ml corn oil (Sigma, C8267) once by intraperitoneal injection on P28, and the lung was collected 4 weeks later.
Tamoxifen-induced Rb-knockout pituitary cancer model
Rosa-creERT2 and Ai14 (tdTomato) transgenic mice were mated to Rbf/f mice63,64 to generate creERT2;tdTomato;Rbf/f mice. Genotypes were determined by PCR analysis using established primers (Supplementary Table 6). tdTomato;Rbf/f littermate mice were used as control. Both creERT2;tdTomato;Rbf/f mice and control mice were treated with 100 mg kg−1 body weight Tamoxifen (Sigma, T5468) in 0.45 ml corn oil (Sigma, C8267) once by intraperitoneal injection on P28 or P56, and the pituitary was collected 2 weeks later.
Tamoxifen-induced Kras
G12D NSCLC model
Rosa-creERT2 and Ai14 (tdTomato) transgenic mice were mated to LSL-KrasG12D mice to generate creERT2;tdTomato;KrasG12D mice. KrasG12D mice is a well-established NSCLC mouse model48. Genotypes were determined by PCR analysis using established primers (Supplementary Table 6). creERT2;tdTomato littermate mice were used as control. To optimize tamoxifen treatment to obtain ectopic division of multiple cell types we treated these mice with 7 doses (0.1, 1, 2.5, 25, 50, 100, 250 mg kg−1 body weight). For 0.1–25 mg kg−1 doses, the overall percent of tdTomato+ cells correlated with dosage but reached a plateau using at higher levels. However, while 0.1–50 mg kg−1 doses induced ectopic division of Spc+ AT2 cells, it did not induce ectopic cell division of Cgrp+ neuroendocrine cells and Ccsp+ club cells, but both 100 and 250 mg kg−1 doses induced ectopic cell division all three cell types. We used the 100 mg kg−1 dose in this study. Both creERT2;tdTomato; KrasG12D mice and control mice were treated once with 100 mg kg−1 body weight Tamoxifen (Sigma, T5468) in 0.45 ml corn oil (Sigma, C8267) by intraperitoneal injection at the indicated age, and lung was collected 2–4 weeks later, as indicated.
Tamoxifen-induced Braf
CA model
Rosa-creERT2 and Ai14 (tdTomato) transgenic mice were mated to BrafCA mice to generate creERT2;tdTomato;BrafCA mice. Genotypes were determined by PCR analysis using established primers (Supplementary Table 6). creERT2;tdTomato littermate mice were used as controls. Both creERT2;tdTomato;BrafCA mice and control mice were treated once with 100 mg kg−1 body weight Tamoxifen (Sigma, T5468) in 0.45 ml corn oil (Sigma, C8267) by intraperitoneal injection at 4 weeks of age, and the lung was collected 2 weeks later.
Staggered EdU–BrdU labelling
For cell cycle duration analysis, animals were injected subcutaneously once with EdU (Sigma, 900584. 30 µg per g of body weight) to label all cells in S phase (DKO retina at P8, Rb−/− pituitary at 4 or 6 weeks of age; Rb−/−;p53−/− lung at E18 and 8 weeks of age; KrasG12D lung at 6, 7, 8 and 13 weeks of age, BrafCA lung at 6 weeks of age). After 2.5 h, BrdU (Sigma, B5002. 100 µg per g of body weight) was injected once subcutaneously. Tissues were collected 0.5 h after BrdU injection, fixed in 4% paraformaldehyde for 1 h (eyes) or 2 h (pituitary, lung) and dehydrated in 30% sucrose for 24 h.
Histology, immunofluorescence and measurements
All collected mouse tissues were embedded in OCT (TissueTek 4583), frozen on dry ice and cut into 12–14 µm sections on Superfrost slides. For S phase labelling, BrdU+ cells were detected using a rat anti-BrdU antibody (Abcam, ab6326, 1:500) or a mouse monoclonal antibody (DSHB, G3G4, 1:1,000). EdU+ cells were detected using Click-iT EdU Alexa Fluor 555 Imaging Kit (Life technologies, C10338). Other antibodies were active caspase-3 (Cell Signaling Technology, 9661,1:500), AP2A (Santa Cruz, SC-8975, 1:500), aPKCι (BD Transduction lab, 610176, 1:500), ARR3 (Millipore, AB15282, 1:500), BRN3 (Santa Cruz, SC-6062, 1:500), calretinin (Santa Cruz, SC-11644,1:500), CCSP (Seven Hills Bioreagent, WRAB-3950, 1:1,000), CGRP (Sigma, C8198, 1:1,000), CRX (C. Y. Gregory-Evans, 1:500), cyclin A2 (Abcam, Ab181591, 1:500), cyclin B1 (Cell Signaling Technology, 4138S, 1:500), galectin 3 (Santa Cruz, SC-19283, 1:500), Ki67 (BD science Pharmingen, 550609, 1:500; ThermoFisher Scientific, 14-5698-82, 1:500), MSHA (Fisher Scientific, AB508MI, 1:200), MYCN (Santa Cruz, SC-791, 1:500), N-cadherin (Santa Cruz, SC7939, 1:500), OC2 (R&D systems, AB6294, 1:500), P21cip1 (Abcam, ab188224, 1:500), p27 (BD Biosciences, 554069, 1:500), phospho-aPKCι/λ (ThermoFisher, 44-968 G, 1:500), phospho-histone H3 (Santa Cruz, SC-8656, 1:500), PKCα (Sigma, P5704, 1:500), PTF1A (Pierre Cordelier, INSERM, France, 1:200), rhodopsin (Santa Cruz, SC-57433, 1:500) and SOX9 (EMD Millipore, MAB5535, 1:500),Spc (Abcam, ab40879, 1:1,000).
Validation of the primary antibodies is provided on the manufacturer’s websites or in the referenced citations: antigen retrieval was performed as described19 by boiling sections in citric acid (H-3300, Vector Lab) or Target Retrieval Solution (S1699, Agilent). Primary antibodies or labelled cells were visualized using donkey anti-mouse, donkey anti-rabbit, donkey anti-rat and donkey anti-goat antibodies conjugated with Alexa-488, Alexa-568 or Alexa-647 (1:1,000; Molecular Probes), or donkey anti-mouse IgG H&L (Alexa Fluor 405) (Abcam, ab175658), DyLight 405 AffiniPure donkey anti-rat IgG (H + L) (Jackson ImmunoResearch, 712-475-153). F-actin was labelled by Alexa Fluor 488 Phalloidin (ThermoFisher Scientific, A12379) or Alexa Fluor 568 Phalloidin (ThermoFisher Scientific, A12380). Nuclei were counter-stained with 4, 6-diamidino-2-phenyindole (DAPI; Sigma).
Labelled cells were visualized and images captured with a Nikon Eclipse Ti or Ti2 laser scanning confocal microscope. Retinal thickness measurements were performed with Nikon NIS-Elements AR 3.10 software. Quantification used horizontal retinal sections containing the optic nerve, and lung/pituitary gland sections, and were analysed with ImageJ (https://imagej.nih.gov/ij/). At least 3 sections per sample and 4–6 samples from different litters were counted.
For whole-mount retinal staining, eyeballs were enucleated and incubated for 30 min in 4% paraformaldehyde. The retinas were incubated at 4 °C with FITC-conjugated IB4 (Sigma L2895) and DAPI in PBS for 1–2 days. After brief washes with PBS, radial cuts were made to divide the retina into 4 quadrants to flatten the retina, and the tissue was mounted with Mowiol. For vascular blood vessel analysis, representative images were analysed with AngioTool (https://ccrod.cancer.gov/confluence/display/ROB2/Home) to assess the vessel covered area (%), average vessel length and mean E lacunarity of the vascular plexus. In brief, at least three 200× magnification images (320 × 320μm field of view per retina) per eye and 6 eyes from the same genotypes of different litters were counted. To compare the vascular density of the DKO retina to the control, we used vascular images taken from a similar depth below the GCL to represent the IVP and DVP for the DKO retina.
Whole-mount F-actin staining
E16 or P0 eyeballs were enucleated and incubated for 30 min in 4% paraformaldehyde in PBS. With a dissection microscope, a circumferential incision was made around the limbus, followed by removal of the anterior segment, lens and vitreous body. The retinas were incubated with Alexa Fluor 488 Phalloidin (ThermoFisher Scientific, A12379, 1:500) or Alexa Fluor 568 Phalloidin (ThermoFisher Scientific, A12380, 1:500) and DAPI in PBS for overnight. After brief PBS washes, radial cuts were made to divide the retina into four quadrants to flatten the retina, and flat retinas were mounted with Mowiol. Immunofluorescent staining was analysed with the Nikon Eclipse Ti laser scanning confocal microscope. The whole retinal area and polarity-defect area were measured with the microscope program.
Senescence-associated β-galactosidase staining
Frozen horizontal retinal sections were stained for β-galactosidase at pH 6.0 using the senescence-associated β-galactosidase staining kit (Cell Signaling, 9860) according to the manufacturer’s guidelines. In brief, 120 μl staining solution was added to each slide, cover-slipped and sealed with rubber cement. The slide boxes were placed in a sealed humid container in a 37 °C dry incubator (no CO2) and incubated for 1–2 days. Colour images were taken with an Olympus BX61 microscope. Positive cells/sections were counted under the Olympus BX61 microscope. For each genotype, at least six retinas were analysed. For each retina, at least three representative sections were counted.
Immune cell detection
P8 eyeballs of p107−/−, α-cre;Rbf/f;p107−/− and α-cre;Rbf/f;p107−/−;Skp2+/− mice were enucleated, and peripheral retinas were dissected in fresh cold HBSS. Dissected peripheral retinas were transferred to 200 μl of cold HBSS per retina. An equivalent amount of Papain solution was added and incubated at 37 °C for 10 min, and the tube inverted gently every 2 min. Next, the digestion solution was discarded by pipetting without disturbing the retina. Mechanical trituration of the retina was performed in 600 μl of neurobasal medium supplemented with 10% FBS by pipetting slowly 10 to 15 times with a P1000 pipette tip. Samples were then DNAse treated for 5 min at 37 °C. Cell suspensions were centrifuged using a swing-bucket rotor at 200g for 5 min. The supernatant was carefully aspirated off the cell pellet, and the pellet was suspended in 1–5 ml neurobasal medium with 1% FBS. Cellular aggregates were removed by straining cells through a 50-μm cell strainer (pluriSelect). Splenocytes were collected by grinding a p107−/− mouse spleen through a 40-μm mesh, and then red blood cells (RBC) were removed by incubating cells for 10 min in RBC lysis buffer (Sigma, 11814389001).
To stain for immune markers, the dissociated retinal cells or splenocytes were washed in FACS buffer (PBS + 1% FBS) and then blocked with mouse Fc block (BD 553141, 1 μg per 106 cells) for 10 min on ice. After washing with FACS buffer, 4 × 105 cells in 200 μl FACS buffer were stained with phycoerythrin (PE) or allophycocyanin (APC)-conjugated anti-B220 (0.5 μg, 12-0452-81), anti-CD3 (0.75 μg, 17-0032-80), anti-CD335/NKp46 (0.75 μg, 12-3351-80), anti-CD45 (0.1 μg, 12-0451-82), anti-CD11b (0.25 μg, 17-0112-81) or appropriate isotype control antibodies (all from ThermoFisher) for 30 min on ice then washed with FACS buffer. Samples were analysed using a Gallios flow cytometer and Kaluza analysis software (Beckman Coulter). Dead cells were excluded using FxCycle violet DNA dye (ThermoFisher). The gating strategy is provided in Supplementary Fig. 8.
Measurement of cell cycle duration
Similar to a prior method39, animals were subject to staggered EdU/BrdU labelling and tissues were collected and fixed (as detailed above). For retinal sections, EdU was detected with the Click-iT EdU Alexa Fluor 647 Imaging Kit first, then co-labelled with antibodies against BrdU (mouse anti-BrdU antibody, DSHB, G3G4), Ki67 (rat antibody, ThermoFisher Scientific, 14-5698-82) to label all dividing cells, and cell-type-specific markers including mature amacrine cells (AP2a), amacrine precursors (PTF1A), Müller glia (SOX9), horizontal cells (OC2), and predominantly neuronal retinal precursors (p21cip1 and MYCN). Matching a prior report74, staining retinal sections from mice subject only to EdU labelling confirmed that the G3G4 mouse anti-BrdU antibody does not cross-react with EdU.
For lung and pituitary sections, EdU was specifically detected using the Click-iT EdU Alexa Fluor 647 Imaging Kit first, followed by an EdU-blocking procedure to prevent cross-reaction with rat anti-BrdU antibody75 (Abcam, ab6326). In brief, after EdU was detected using the Click-iT EdU Alexa Fluor 647 Imaging Kit, slides were treated with 2 mM azidosulfide (Sigma, 244546) in the Click-iT buffers for 30 min. Staining sections from mice subject only to EdU labelling confirmed that the anti-BrdU antibody did not cross-react with EdU. After the EdU-blocking procedure, slides were co-labelled with antibodies against BrdU (rat anti-BrdU antibody, Abcam, ab6326), Ki67 (mouse antibody, BD science Pharmingen 550609) to label all dividing cells, and cell-type-specific markers including lung neuroendocrine cells (CGRP, rabbit, Sigma, C8198), lung club cells (CCSP, rabbit, Seven Hills Bioreagents, WRAB-3950), lung alveolar type II cells (SPC, rabbit, Abcam, ab40879), and pituitary melanotropes in the intermediate lobe and corticotropes in the anterior lobe (MSHA, Fisher Scientific, AB508MI), and imaged by confocal microscopy.
ImageJ (Fiji, version 1.54g) was used to manually count EdU/BrdU-labelled cells in 60× images using the image\color\channels tool. For cell-specific cell cycle data, we first counted the number of cell types of interest (for example, CGRP+ neuroendocrine cells). Next (or first in cases where no cell type-specific marker was used), we added the Ki67 channel to count dividing cells (CGRP+; Ki67+), then we added the EdU channel to count how many dividing cells were EdU-positive (CGRP+; Ki67+; EdU+). Next, we added the BrdU channel to count how many dividing cells were BrdU-positive (CGRP+; Ki67+; BrdU+). After that, we combined EdU and BrdU data with Marker/Ki67 to count marker+Ki67+BrdU−EdU+ cells. Counting each image was performed three times to ensure accuracy. Functions used to calculate Tc and Ts are illustrated and explained in detail in Supplementary Fig. 3. All the raw counts used in Tc and Ts calculations are provided in Supplementary Table 7. Examples of images used for counts are provided in Supplementary Figs. 4–7.
The number of EdU+;BrdU− cells forms the denominator of Tc and Ts calculations (Supplementary Fig. 3), thus if some of these cells divide this denominator would increase, artificially decreasing Tc and Ts. Prior publications assume that no or very few EdU-labelled cells divide, and that the EdU-only cells have reached G2/M but not beyond. We performed additional assays to examine this assumption.
First, we labelled P8 Rb/p107 DKO mice with EdU for 1 h, 1.5 h, 2 h, 3 h, 4 h, 5 h or 6 h, then stained retinal sections for EdU, phospho-histone H3 (PH3) and DAPI (Supplementary Fig. 9a). H3 phosphorylation begins in late G2 with chromosomal condensation, but is most prominent in early-mid M phase, and H3 dephosphorylation commences in late anaphase/early telophase76, thus PH3 is widely used as a late G2/M marker. If G2/M was <3 h, all cells in S phase at the time of EdU labelling would have reached M phase by then, and the EdU+ cells that were labelled in early-mid S phase would continue to enter G2/M at later times (S phase in DKO P8 retina is ~25 h, Fig. 4c), ensuring a long plateau of 100% EdU+;PH3+ double-labelled cells beyond 3 h. Conversely, if G2/M is >3 h, only a fraction of PH3+ cells would also be EdU+, which would continue to rise with time, eventually reaching the 100% plateau. As expected, the fraction of PH3+ cells that were also EdU+ increased over time, confirming the movement of prior S phase cells through G2 into M (Supplementary Fig. 9b). No double-labelled cells were detected at 1 h or 1.5 h, a tiny fraction was seen at 2 h, which continued to increase only reaching 100% at 6 h. At 3 h, the time point used in our Tc measurement studies, only 23.9% of PH3+ cells were EdU+ (Supplementary Fig. 9b). These data suggest that 3 h is insufficient for EdU-labelled cells in the P8 DKO retina to divide.
Second, we also performed EdU/PH3/DAPI triple labelling in sections from Rb pituitary, Rb/p53 lung, Kras lung, and Braf lung cancer models used in the paper (Supplementary Fig. 9c). The fraction of M phase cells that were EdU+ after 3 h was ~30–50%, similar in range to the DKO retina, and well below the 100% expected if EdU+ cells could complete mitosis in 3 h (Supplementary Fig. 9d). Thus, 3 h also appears insufficient for EdU-labelled cells to divide in these cancer models.
Third, as an additional test in retina, P8 DKO mice were exposed to EdU at 0 h, then BrdU from 2.5–3 h and retinal sections were stained for EdU. A cocktail of three rabbit antibodies targeting cyclin A2, cyclin B1, and PH3 to label S, all G2 and all M phase cells (Supplementary Fig. 9e). Any EdU-only cells that traverse M into G1 would be EdU+ but negative for all the other markers (cyclin A2, mid/late S phase and G2; cyclin B1, G2 and early M phase; PH3, late G2 and M phase). Notably, all the EdU+ cells co-labelled with the A2/B1/PH3 antibody mix, and no cells were positive only for EdU (Supplementary Fig. 9f). This result confirms that EdU-labelled cells do not divide in a 3 h window.
Single-cell RNA sequencing
Retinal dissociation
P8 eyeballs of α-cre;Rbf/f;P107−/− (DKO, tumour-prone) and α-cre; Rbf/f; P107−/−; Skp2+/− (DKO-Skp2+/−, tumour-resistant) mice were enucleated, and peripheral retinas (α-cre expression areas) were dissected in fresh and cold HBSS. Dissected peripheral retinas were then transferred to 200 μl of cold HBSS per retina. An equivalent amount of Papain solution (for 1 ml, 700 μl reagent grade water, 100 μl of freshly prepared 50 mM l-cysteine (Sigma), 100 μM 10 mM EDTA, 10 μM 60 mM 2-mercaptoethanol (Sigma), and Papain added to 1 mg ml−1 (Worthington)) was added and incubated at 37 °C for 10 min, the tube was inverted gently every 2 min during the incubation. After the incubation steps, the digestion solution was discarded by pipetting without disturbing the retina. Mechanical trituration of the retina was performed in 600 μl of neurobasal medium supplemented with 10% FBS by pipetting slowly 10 to 15 times with a P1000 pipette tip. Samples then were subjected to DNAse treatment (5 μl DNAse I (RNAse-free Recombinant DNAse I; Roche) for every 1 ml of dissociation solution) for 5 min at 37 °C. Cell suspensions were then centrifuged using a swing-bucket rotor at 200g for 5 min. The supernatant was carefully aspirated off the cell pellet, then resuspended in 1–5 ml neurobasal medium with 1% FBS. Cellular aggregates were removed by straining cells through a 50-μm cell strainer (pluriSelect).
Single-cell library preparation and sequencing
Single-cell gene expression libraries were prepared using the Single Cell 3′ Reagent Kit v2 (10x Genomics) according to the manufacturer’s protocol. Libraries were sequenced using the HiSeq3000 (Illumina) with the 10x Gene Expression recommended parameters (read 1, 28 cycles and read 2, 100 cycles).
CellRanger pre-processing
Raw data were processed through the CellRanger pipelines (10x Genomics, V2.2.0). For each run of DKO and DKO-Skp2+/− genotypes, reads were quantified into UMI gene–barcode matrix using the mouse reference index provided by 10x Genomics (refdata-cellranger-mm10) and default parameters of the count pipeline to filter out low-quality cell barcodes. This process resulted in 3,621 estimated cells with an average of 52,983 reads and a median of 2,884 genes per cell for the DKO sample and 5,196 estimated cells with an average of 31,520 reads and a median of 1,873 genes per cell for the DKO-Skp2+/− sample. Then, matrices for individual runs were aggregated together into a single gene–barcode matrix with the CellRanger’s aggr pipeline to avoid artefacts that may be introduced due to differences in sequencing depth when comparing the two genotypes. The aggregation kept 74.3% reads from the DKO sample and 100% reads from the DKO-Skp2+/− sample and ended with 8,817 estimated cells with an average of 34,749 reads and a median of 1,941 genes per cell.
Identification of non-retinal cell types
The cloupe files generated by CellRanger were viewed using the 10x Genomics Loupe Browser and cells in clusters were explored by their over-expressed genes and expression of typical retinal, immune and astrocyte cell marker genes. We defined clusters as immune for their outstanding expression of marker genes Aif1 and Lgals9, and astrocyte cells by marker gene Aqp4. The proportions of immune and astrocyte cells in total cells were compared between DKO and DKO-Skp2+/− genotypes. For further analysis of retina cells, these non-retinal cells were excluded.
Quality control and normalization
After removing non-retinal cells, data was further processed and analysed mainly by Scanpy Python toolkit (https://github.com/scverse/scanpy) and Seurat R toolkit (https://github.com/satijalab/seurat). For downstream analysis, low-expression genes and poor-quality cells were further filtered out from each single-cell dataset. After filtering, only genes expressed in >3 cells and cells expressing >500 genes and less than 15% of mitochondrial genes were retained, with 3,501 cells in DKO and 5,019 cells in DKO-Skp2+/− dataset. The retained data were normalized, and log-transformed by Scanpy’s normalize-total and log1p, respectively. The aggregated data were filtered by only keeping the cells retained in each individual sample after the above filtering and genes with >10 counts in total. This resulted in 8,520 cells, including 5,019 DKO-Skp2+/− and 3,501 DKO retinal cells, and 15,731 genes. Then the aggregated data were normalized and log-transformed following the same methods as above.
Assigning cell cycle phases
To identify cycling cells and the effect of cell cycle heterogeneity on the data, each cell was assigned cell cycle scores and phases using Scanpy’s function score_genes_cell_cycle. A list of cell cycle marker genes, including markers for S and G2M phase77 were used for the scoring, which calculates the difference of mean expression of the given cell cycle genes and the mean expression of randomly selected reference genes from binned gene pools that match the distribution of the given genes’ expression. The human gene names were converted to mouse gene names by biomaRt package. Based on its cell cycle scores, a cell is predicted to be in G2M, S or G1 phase. Cells expressing neither S nor G2M genes are likely not cycling (G0) or in G1 phase.
Detection of doublets
To detect if any cells were grouped together as a cluster by artefacts of the problematic doublets or multiplets, where two or more cells receive the same barcode and result in a hybrid transcriptome, the python package “scrublet”78 was used to calculate doublet score and predicted doublets for each sample and possible artefact clusters by default parameters and expected doublets rate—DKO, 0.03; and DKO-Skp2+/−, 0.04—based on the number of captured cells. Raw data matrices for each individual sample were used for this analysis. The annotation of the detected doublets for cells were transferred onto the aggregated data.
Clustering and identifying major retinal cell components
To minimize the effects of cell cycle heterogeneity and to emphasize clustering based on genes related to cell lineage, the cell cycle was regressed from the previous normalized expression matrix. Both scores for G2M and S phase were regressed out with the regress_out function of Scanpy. After scaling, the top 2,000 highly variable genes were selected for the principal component analysis to reduce the dimension of data. The neighbourhood graph of cells was computed with the top 50 principal components, and then, the graph was embedded into two dimensions using UMAP. Cell clusters were identified by the Leiden graph-clustering method79, which directly clustering the neighbourhood graph of cells.
To annotate the retinal cell identity of each cluster, we associated the clusters with retinal cell types based on scores of known retinal cell-type-specific genes (Extended Data Fig. 4h). The scores of each cell type were calculated for each cell using the Scanpy’s score_genes function with the same algorithm of the score_genes_cell_cycle. A cluster was assigned to a cell type with the highest average score and percentage of cells detected with positive scores within the cluster. The cell-type annotated clusters were further refined and verified by highly differential expressed genes for each cluster. The DEGs were identified for each cluster against the rest of the clusters using the rank_genes_group function with Wilcoxon rank-sum test. Of them, marker genes for each cluster were further filtered by the filter_rank_genes_groups function with default parameters. Expression of the top 10 marker genes for each cluster were compared between mouse normal retinal development and these samples by UMAP visualization. Using the normal mouse retina development scRNA-seq data80 As a reference, we also identified each individual cell for its cell type with the ingest function of Scanpy, which fit a model on the reference data and used it to project new data with cell annotations. This mapping was applied using data for each individual sample, and mapped cell types were transferred to the aggregated data. The cell cycle effects were regressed out from the reference data and genotype data before mapping.
Top genes that distinguished particular cell types included: Apoe, Müller glia cluster (Mu); Rom1, photoreceptors (PR); Ebf1, amacrine precursors (AmP); Pcsk1n, mature amacrine cells (AmM); Fbxo5, rare remaining mitotic progenitors (ProgM); Isl1, bipolar cells (BiP); and Otx2, Neurod1 and Neurod4, neurogenic (Neu) cluster80,81,82,83,84,85,86 (Fig. 3b, Extended Data Fig. 4g and Supplementary Table 2).
Differential gene expression between two genotypes within each cluster
To identify DEGs between two genotypes within each cluster, the function FindMarkers from the Seurat package was used with the MAST test for two condition comparisons. Significant DEGs were selected as those with adjusted P values less than 0.1, average log2 fold change higher than 0.25 and percentage (proportion) of cells with expression within a cluster larger than 0.1. The enrichment of CDK2-associated genes37 and cell cycle genes expressed in S and G2M phases in the DEGs were evaluated by the hypergeometric test phyper in R. The Bioplanet terms enriched in the DEGs were also identified using the online tool Enrichr (https://maayanlab.cloud/Enrichr/)87.
Western blotting
Peripheral mouse retinas were homogenized with a 30-gauge needle 5–10 times in 1× cell lysis buffer (Cell Signaling 9803) with 0.1 mM PMSF, 1μg ml−1 aprotinin and 1μg ml−1 leupeptin. Proteins were separated by SDS–PAGE, transferred to nitrocellulose membrane, and analysed using ODYSSEY Infrared Imaging System (LI-COR) with antibodies against active caspase-3 (Cell Signaling Technology 9661, 1:200), p27 (554069, BD Biosciences, 1:200), SKP2 (SC-74477; Santa Cruz, 1:1,000) and β-actin (A5441, Sigma, 1:2,000).
Statistical analysis
Sample sizes were chosen based on power analysis and common practice in mouse experiments. Sample sizes (n) were estimated by the formula n = [2(Zα + Z1–β)2]/SES2. Type I error (α) is set as 0.05, type II error (β) is set as 10–20% and is a two-sided effect. The SES (Cohen’s d) is the standardized effect size, equal to the ES (effect size) divided by the pooled s.d., so it is the magnitude of the difference between the means of two groups in s.d. units88. For animal experiments, SES of 1.1×, 1.5× and 2.0× s.d. are suggested to represent small, moderate and significant responses, respectively88. According to the formula above, 4–7 mice are required to detect effect with 80% power, and 6–10 mice are needed to detect effects with 90% power. Thus, 4 or more typically 6 mice were used for each experiment to detect impact with 80–90% power. All data are presented as mean ± s.d. Kaplan–Meier survival curves and statistical analysis were performed using GraphPad Prism software. An unpaired Student’s t-test was used to compare two groups. One-way ANOVA followed by Bonferroni correction was used for multiple comparisons. The P values for Kaplan–Meier curves were calculated using the log-rank (Mantel–Cox) test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.