Capture and care of moths
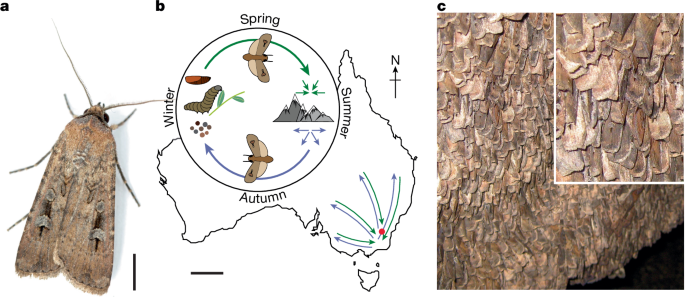
Bogong moths (A. infusa) of both sexes were caught in the wild during their autumn and spring migrations (2019 and 2018) using a LepiLED insect light (www.gunnarbrehm.de), or a vertical beam search light (model GT175, Ammon Luminaire Company), placed in front of a white sheet suspended between two trees. Almost all of the animals were caught near the Mount Selwyn Snowfields (southeast New South Wales, Australia: 35.914° S, 148.444° E; elevation, 1,600 m), which is approximately 70 km north-northeast of the nearest aestivation cave in the Main Range of the New South Wales Alps. Thus, to reach these caves in spring, these moths (a tiny subset of all moths travelling to the mountains in a multitude of directions from across southeast Australia) would be expected to fly south-southwest in spring, and returning moths might be expected to travel north-northeast in autumn (which agrees with our behavioural results). A few animals were also caught near Thredbo (Dead Horse Gap, southeast New South Wales, Australia: 36.524° S, 148.260° E, elevation 1,580 m). These moths were used for electrophysiology only. Each captured moth was transferred to its own plastic container to isolate it from influence by other moths. After capture, moths were transported to the testing site Glenhare, a rural property near Adaminaby New South Wales (36.040° S, 148.864° E; elevation, 1,250 m), fed with 20% honey solution (in water) and stored in a cool and sheltered place (exposed to the natural light cycle) to recover from stress induced by capture.
Laboratory for controlled indoor experiments
A purpose-built ferromagnetic-free laboratory located at Glenhare, Adaminaby (built on a concrete slab reinforced with fibreglass and constructed entirely from non-magnetic materials) housed the indoor behavioural and electrophysiological experiments (Extended Data Fig. 1c). Each experimental apparatus (behaviour and electrophysiology) has its own dedicated earth separated from the mains earth (through a 6-mm thick, 30-mm wide and 12-m long copper strap dug into the ground below the concrete slab). Background levels of radio-frequency disturbances at this rural site are extraordinarily low4. All of the experiments were performed on dark-adapted moths in darkness at night (beginning at least 1 h after sunset). Darkness was achieved with black-out blinds (to remove residual starlight and moonlight from outside) and dark cloth around the experimental apparatus (to shield from the minimal stray light emitted from the equipment).
Non-magnetic electrophysiological apparatus
The non-magnetic electrophysiological apparatus (such as table and animal mounts; Extended Data Fig. 1f,g) was constructed from Thorlabs aluminium optomechanical components using high-grade stainless-steel fasteners. Vibration isolation between the aluminium pillar legs and the aluminium bread board table (on which the moth and manipulators were mounted) was provided by four high-grade stainless steel Stillpoints Ultra 6 (with Ultra base) isolators (Stillpoints). The moth was mounted (see below) onto a pillar attached to the bread board table, and a custom-built non-magnetic Sensapex piezo micromanipulator (Sensapex Oy, Oulu), also attached to the pillar, was used to move and advance a glass microelectrode. A removable circular UV-transmissive Perspex disc (diameter, 250 mm; thickness, 5 mm), covered in a layer of UV-transmissive diffusing paper (Lee Filters 251 1/4 white diffuser) and mounted 127 mm above the moth, was used for projection of celestial visual stimuli (see below). The electrophysiological apparatus was placed at the centre of a computer-controlled, double-wrapped41 three-axis (3D) Helmholtz coil system custom built in aluminium and copper (University of Oldenburg workshop; outer coil diameters; x, 900 mm; y, 835 mm; z, 775 mm) to create a nulled magnetic field (Extended Data Fig. 8) around the experimental moth. These coils were mounted onto the experimental table holding the moth and manipulators. The coil systems were powered by constant-current power supplies (Kepco, BOP 50-2M) and the current running through the coil systems was controlled through High-Speed USB Carriers (USB-9162, National Instruments) and custom-written codes in MATLAB (v.2019a and 2022b, MathWorks). Further details were reported previously3,4. Before each experimental session, Meda FVM-400 magnetometer measurements ensured that the magnetic field was nulled within the apparatus (Extended Data Fig. 8b).
Non-magnetic behavioural apparatus
The non-magnetic behavioural apparatus (Extended Data Fig. 1h,i) consisted of a modified Mouritsen–Frost flight simulator3,4,5,42 used to record the virtual flight path of tethered migratory Bogong moths. In brief, each flight simulator consisted of a cylindrical Perspex arena (diameter, 50 cm; height, 35 cm) placed vertically onto an aluminium table with a clear Perspex top within a 3D Helmholtz coil system (as described above, but with coil outer dimensions: x, 1,245 mm; y, 1,300 mm; z, 780 mm). Again, the nulled magnetic field conditions within the coils were carefully monitored using the Meda FVM-400 magnetometer (Extended Data Fig. 8a). The arena walls were covered with two layers of black felt. An optical encoder (E4T Miniature Optical Kit, US Digital) was mounted in the middle of a UV-transmissive Perspex disc (diameter, 50 cm; thickness, 0.5 cm), which was placed on top of the arena like a lid. A layer of UV-transmissive diffusing paper (Lee Filters 251 1/4 white diffuser) was placed on top of the disc (and served as a screen for dorsal projection of celestial stimuli; see below). A fine vertical tungsten rod (the encoder shaft: diameter, 0.5 mm; length, 153 mm), inserted into the axial centre of the optical encoder, extended downwards into the arena and allowed the attachment of tethered flying moths (see below). We used the encoder manufacturer’s software (USB1 Digital Explorer 1.07, US Digital) to continuously record the moth’s heading relative to geographical north (gN), therefore allowing us to reconstruct the moth’s virtual flight path in the presence of celestial visual cues. An LED projector (ASUS S1 Mobile), neutral density (ND) filters (optical density between 4 and 5 log units) and a mirror placed at 45° under the Perspex tabletop were used to project a dim moving (10 mm s−1) pattern of optic flow onto a screen (Lee Filters 251 1/4 white diffusing paper) placed beneath the arena and therefore below the tethered flying moth (Extended Data Fig. 1d). The direction of the optic flow was controlled by custom written software (M. York), which coupled the encoder system (USB1 or USB4 encoder data acquisition USB device, US Digital) through a feedback loop. Thus, the optic flow would always move from head to tail below the moth, instantaneously changing direction as the moth changed direction. The mean radiance of the optic flow at the location of the performing moth was 2.06 ± 0.19 × 109 photons cm−2 s−1 sr−1.
Stimulation with natural starry skies
A natural moonless starry austral night sky, in both the electrophysiological and the behavioural rigs, was projected using a downward pointing projector (behavioural rig: LED projector ASUS S1 Mobile; electrophysiological rig: Sony MP-CL1A laser projector; spectra of both projectors are shown in Extended Data Fig. 9d). Each projector was mounted sufficiently high above the moth in each rig to provide clear and correctly sized dorsal images of the night sky. To avoid unwanted stray light, each projector was housed in a custom-built 3D-printed plastic box featuring an opening in front of the lens and ventilation slits above the projector.
The free software Stellarium43 was used to simulate the moonless starry night sky over Canberra (about 80 km from Adaminaby as the crow flies) at 21:30 on four respective dates: 1 October 2018 (spring 2018), 21 March 2018 (autumn 2018), 21 October 2019 (spring 2019) and 27 February 2019 (autumn 2019). Screenshots (screen resolution 7,480 × 720 pixels) of these simulated starry skies were taken, cut in a circular shape using CorelDRAW X5 and saved as PNG files (300 dpi) to create the stimulus images (Fig. 3a,b and Extended Data Fig. 9a). Subtended at the moth, celestial images projected onto the circular screens in each rig (see above) provided 160° (behavioural rig) and 100° (electrophysiological rig) fields of view of the starry night sky centred on the zenith. Celestial images contained grey level values ranging from 4 (darkest) to 255 (brightest, on a scale of 0–255), with an average grey level of 62. The quality of the night sky provided by these images was comparable to that provided by the natural rural night sky at Glenhare (as measured with a Unihedron Sky Quality Metre; Extended Data Table 1). Before each experiment, the PNG files were opened using IrfanView64 on a PC using a screen resolution of 1,280 × 720 pixels. The PC was connected through an HDMI cable to the projector. The projected size of the circular sky matched the diameter of the circular screen in each rig.
Before each experiment, we ensured that the projection was centred accurately and that its light intensity was reduced to starlight levels using ND filters (the behavioural rig is shown in Extended Data Fig. 9b). In the case of the electrophysiological rig, two 1.2 log unit ND filters were inserted into the light path to dim the image to starlight levels (Extended Data Fig. 9c). The projection of the starry sky was initially set to its natural orientation relative to geographical north or flipped along its vertical and horizontal axes to test the moths under a 180° rotated sky.
As the projectors did not emit UV light, we installed a custom-made LED-ring (built by T. McIntyre; diameter, 120 mm; inner diameter, 50 mm) featuring eight UV LEDs (LED370E Ultra Bright Deep Violet LED, Thorlabs) in front of the projector. The brightness of the LED-ring was controlled using custom written software using MATLAB (v.2019a and 2022b, MathWorks) and several layers of ND filters that were fixed in front of the LED-ring to bring the UV intensity into a quasi-natural range (the behavioural rig is shown in Extended Data Fig. 9d).
Upward light intensity and spectrum measurements of the starry night sky were made underneath the projection screen in each rig with the probe located at the position of the moth (Extended Data Fig. 9b–d). The light metre used was a calibrated Ocean Optics QE65PRO Spectrometer (Ocean Optics).
Stimulation with randomized starry skies
To create randomized starry skies for experiments in autumn 2018 and spring 2018, the positions of all individual pixels of the natural night sky stimulus image were reassigned randomly to new positions, and the resulting randomized images were likewise saved as PNG files (Extended Data Fig. 9a). These featureless stellar conditions provided an identical stimulation intensity but provided no celestial spatial information. The randomized starry skies used during spring and autumn 2019 were improved by randomizing groups of pixels containing individual stars, therefore retaining the stars but removing spatial variations in the night sky (such as the Milky Way) that could be used for orientation (again maintaining identical intensity). Here, the stimulus image (of autumn 2019) was subdivided into squares with a size of 13 × 13 pixels, as the brightest star in the image had roughly these dimensions. The positions of these squares were now randomly reassigned and the resulting image was saved as a PNG file. A final improved randomized stimulus (used during spring 2019) was generated from the test stimulus by randomizing the positions of the individual visible stars. This was achieved by first detecting the position and size of each star in the test stimulus using a multiscale Laplacian of a Gaussian convolution of a greyscale version of the test stimulus, followed by local maxima detection. The resulting spatial information was then used to extract and save the image of each star from the natural night sky stimulus, before replacing them on a new background image, with a uniform colour and intensity equal to that of the mean of all pixels in the test stimulus that were not part of a star. This way, the location of the stars on the randomized image could be drawn from any desired distribution. In this case, a uniform distribution was used for the location of all but the brightest star, which was placed in the centre of the image.
Stimulation with artificial Milky Way cues
To test the selectivity of central brain visual neurons to various parts of the Milky Way during electrophysiological experiments, we stimulated cells with artificial compass cues that, like the starry sky stimulus, filled the projection screen above the moth (Fig. 4d). These cues mimicked aspects of the autumn Milky Way: its brightest region around the Carina nebula (revolving dot) and its stripe-like shape (rotating bar). The bar length and width were chosen to mimic the main stripe of our natural Milky Way stimulus. The intensities of these artificial stimuli were also adjusted to provide a good mimic of the intensities of the stars and the dark background sky in our natural stimulus (see above) and had the same mean grey level (61 ± 1 for values between 0 to 255, darkest to brightest).
Apparatus for behavioural experiments under an open sky in a natural landscape
Two ferromagnetic-free Mouritsen–Frost flight simulators (of the same type used in the laboratory) were placed on a hilltop at Glenhare and used to record the heading directions of tethered migratory Bogong moths experiencing the full local surrounding landscape and the entire dome of the natural sky. To achieve this, each flight simulator arena consisted of a transparent UV-transmissive Perspex cylinder (again with diameter, 50 cm; height, 35 cm), placed vertically onto an aluminium table (Extended Data Fig. 1a,b). The table top was also made of transparent Perspex. The two flight simulators (and their tables) were placed around 15 m apart. A tethered moth was connected to an optical encoder suspended at the centre of the open top end of each arena by a thin horizontal UV-transmissive Perspex arm. The two arenas were controlled from computers that were operated from within a black light-tight cubical tent that was placed midway between the two arenas, approximately 7 m from each. The tent was therefore a landscape feature that the moths could potentially see during their tethered flights.
Behavioural procedures
Indoor behavioural procedures
Most behavioural procedures used in this study have been previously described4,5. Before attachment of tethering stalks, moths were chilled in a freezer for 5–10 min to immobilize them. The scales on the moth’s dorsal thorax were removed by suction using a micro-vacuum pump (custom built by B.F.). Afterwards a thin vertical tungsten stalk (which is ferromagnetic free), fashioned at its end to create a small circular footplate, was glued to the dorsal thorax using contact cement while being restrained by a weighted-down plastic mesh. Moths were tested on the day of stalk attachment.
Shortly before sunset, UV-transmissive Perspex boxes holding individual stalked moths were placed onto an elevated outdoor location and provided with a clear view of the western sky and the setting sun (and the skylight polarization pattern), in case these cues were important for calibrating compass mechanisms (as found for the magnetic compass of birds44,45,46). After sunset, the moths could also see the stars (and the celestial rotation).
For tethering within the behavioural arena (performed in dim red light to maintain a dark-adapted visual state), the arena lid holding the optical encoder was lifted and the tungsten stalk of a vigorously flying moth (held with medical forceps) was attached to the bottom end of the encoder shaft through a 1.5 cm length of thin rubber intravenous medical tubing that connected the stalk to the shaft (Extended Data Fig. 1e). Once the arena lid was returned to the arena, this coupling enabled the moth to rotate freely around its yaw axis and choose any flight direction. Once the moth was mounted in the arena, it was gently pointed manually towards geographical north. The heading direction count was then reset, the moth was released and the optical encoder was enabled to register the flight heading direction of the moth under a given night sky condition (projected on the arena lid) at a sampling rate of 5 Hz (and a horizontal resolution of 3°). Each moth was tested for exactly 5 min in each stimulus condition.
Moths chosen for analysis were required to fulfil three ante hoc criteria, two before the experiment and one during the experiment: (1) the tethering stalk was perfectly vertical; (2) wing flapping was vigorous and its amplitude was large and equal for both wings (indicating that the contact cement had not interfered with the wings); and (3) that the moth flew continuously for the full 5 min. For the last criterion, if a moth stopped flying, the arena was gently tapped in order to stimulate the moth to continue flight behaviour. A moth that stopped flying four times was rejected and the recording aborted. For the field seasons of spring 2018 and 2019, as well for Autumn 2019, the percentages of moths that were aborted due to failing to meet these criteria were 2 out of 59 or 3.4% (Spring 2018), 25 out of 137 or 18.3% (Spring 2019), and 35 out of 76 or 46.1% (Autumn 2019). The high rejection rate during Autumn 2019 may have been due to the unusually wet and cold weather that occurred during this season. Moths often behave erratically on rainy or stormy nights, and even on days before and after such nights.
Outdoor behavioural procedures
The same behavioural methods (and criteria) that were used indoors were also used outdoors. The goal of these experiments was to understand how migratory Bogong moths deal with natural skies (in particular the nightly movements of the stars and moon, as well as cloud cover) while experiencing a normal geomagnetic field and the local surrounding landscape under natural illumination. Experiments were performed under clear starry skies during autumn 2023 (over three nights during the last week of March), as well as at two times of night, to test whether the migratory orientations of moths were affected by the nightly movements of the stars and moon (which was approximately half-full): (1) between 20:32 and 21:06 (about 1.5 h after sunset), and (2) between 23:25 and 23:59. The same moths were used for orientation measurements at both times, and moths that were flown in one arena at the earlier time (and saw the black tent on their eastern side) were flown in the other arena at the later time (and now saw the black tent on their western side) and vice versa. Moreover, there was a stand of trees close to the arena on the eastern side of the tent, and single trees close to the arena on the western side of the tent. Thus, other panoramic landmarks (apart from the tent) differed markedly in their spatial positions from within the two arenas. Between earlier and later experiments, tethered moths were kept isolated and in the dark in a suitcase that was warmed with hot water bottles. Experiments were also carried out on a fourth completely overcast night that totally covered the stars and moon (between 21:12 and 21:48).
Indoor electrophysiological procedures
To maximize the success rate of the demanding intracellular recordings during the short migratory season, these experiments were performed both in the afternoon and during the natural nocturnal flight time of the moths. For afternoon experiments, we removed the two 1.2 log unit ND filters in front of the projector lens (as described above) to generate a starry sky projection around 250 times brighter than the one used at night (to account for the circadian-rhythm-induced light-adapted state of the moths). For night experiments, the ND filters were reinserted. No obvious differences were found in results obtained in the two situations. The moths were mounted onto a custom-made 3D-printed animal holder and immobilized using wax. The antennae were fixed to the front of the head with a drop of wax, and a square piece of cuticle was removed from the head capsule to expose the brain. The neural sheath was digested with Pronase (Sigma-Aldrich) for about 30 s and then carefully washed. It was then removed using a pair of fine forceps. A second small hole was cut into the cuticle above the proboscis muscle and a chlorinated silver wire was inserted into this muscle to serve as reference electrode.
Glass electrodes were pulled from borosilicate glass on a P-97 Flaming-Brown micropipette puller (Sutter Instrument), and had a typical resistance of 50–100 MΩ. The electrode tip was filled with Neurobiotin solution (4% Neurobiotin in 1 M KCl, Vector Laboratories), and the remainder of the electrode was filled with 1 M KCl. Electrodes were moved into position with a non-magnetic Sensapex micromanipulator. Signals were amplified using a BA-03X intracellular amplifier and headstage (NPI Electronic), and were then digitized using a CED Micro 1401-3 (Cambridge Electronic Design) and recorded with Spike2 software (v.8.03, Cambridge Electronic Design). Stimulus control signals from MATLAB (v.2019a and 2022b, MathWorks) were simultaneously recorded in Spike2. During the recording, the brain was kept hydrated through regular application of moth ringer solution47.
Moths were mounted under starry skies that were naturally oriented before sky rotation (that is, had the same orientation as the stars outside the laboratory). In earlier experiments, the initial heading orientation of the mounted moth was north relative to the stars and, in later experiments, the initial heading orientation south; however, greatest ease of access to the brain was eventually found for an eastward heading. The action potential spike trains obtained for a 360° rotation of the sky were corrected according to the initial heading orientation of the moth so that spike trains obtained across all experiments were comparable (with a correction angle of 0° applied for an initial northward orientation, 90° for an eastward orientation and 180° for southward orientation). Impaled cells were stimulated with dorsally projected images of the natural starry night sky, or control images of randomized stars (see above). Cells were also stimulated with bars and dots that mimicked different parts of the Milky Way (see above). All of these projected images (natural and randomized stars, artificial Milky Way stimuli) were rotated 360° at 30° s−1 to 45° s−1, with a 2 s break between clockwise and anticlockwise rotations, using custom-written MATLAB code (v.2019a and 2022b, MathWorks). Between rotations, a neutral grey background image was presented that had the same average grey value as the starry sky/control images.
We targeted an area of the central brain in which we expected to find both CX and lateral complex neurons, as well as optic lobe neurons traversing the brain in the posterior optic tract. Neurons that clearly did not respond to an initial sky rotation were immediately discarded and no recording was saved. 79 neurons were assessed as potentially responding to the stimulus and, of these, 28 (35%) met the inclusion criteria of a unimodal or bimodal response profile. The remaining 51 neurons were classified as uniform in their response to stellar rotation (that is, showed no obvious response) and were therefore excluded from the analysis.
After recording from a suitable cell, a positive current (range: 1–3 nA for 3 min) was applied to the electrode to inject Neurobiotin into the cell. The electrode was then removed and the brain was dissected out of the head capsule. Brains were fixed in 4 °C overnight in paraformaldehyde solution (4% PFA in phosphate buffer) and then washed in 0.1 M PBS (4 times for 15 min). During washing, the retinas were removed. Brains were then incubated with streptavidin–Cy5 (Jackson Immuno Research, 1:1,000 in PBS with 0.3% Triton X-100) at 4 °C for 3 days and kept in the dark from this point onwards. After incubation, the brains were washed in PBS-Triton X-100 (6 times for 20 min) and PBS (2 times for 20 min) and then dehydrated in an increasing ethanol series (50%, 70%, 90%, 95% and twice at 100%). Brains were then transferred to a fresh mixture of methylsalicylate and ethanol (1:1) and, after 15 min, were left to clear in 100% methylsalicylate for 75 min. The cleared brains were mounted in Permount (Thermo Fisher Scientific) mounting media between two coverslips and left to dry for at least 2 days.
Brain samples were scanned with the 633 nm laser of a Leica SP8 confocal microscope and viewed with a ×20 oil-immersion objective (Leica Microsystems). For optimal resolution, the scan settings were set to 1,024 × 1,024 pixels, 12-bit pixel depth, 3 times line accumulation and 400 lines per s in the photon-counting mode of the hybrid detector. Neurons and relevant neuropils were then reconstructed in Amira v.5.3 (Thermo Fisher Scientific) and registered into the Bogong moth standard brain22,23.
Data analysis
The data used for the analyses described below are available online48. As there were no statistical differences in results obtained from male and female moths, the results were pooled.
Indoor laboratory behaviour
The behavioural analysis used in this study has been previously described4,5. As mentioned above, the encoder software (USB1 Digital Explorer v.1.07, US Digital) recorded the instantaneous heading directions of a tethered flying moth every 200 ms (5 Hz) and saved these values in a text file. We used custom-written MATLAB code (v.2019a and 2022b, MathWorks) to visualize the virtual flight paths of all tested moths and calculated a mean orientation vector based on each virtual flight path. Each vector for each moth in the circular plots (Fig. 3c–f) encodes the mean orientation direction of a moth’s individual recorded flight path as well as its r value (that is, length, or directedness, of the flight path vector). To take advantage of the extra information in our data arising from the fact that the flight trajectories of moths not only had a mean direction (as used for a classic Rayleigh test49) but also a mean directedness (vector length), we used the circular statistics software Oriana (v.4 (2011), KCS) and Excel (Microsoft Office 2019, Microsoft) to apply a one-sided Moore’s modified Rayleigh test4,50,51 with Bonferroni correction for multiple comparisons (Supplementary Table 1). The R* value encodes the directedness of a population of tested moths and reveals the likelihood that the combined flight direction of these moths—each with its own direction and directedness—differs significantly from random.
To confirm that the two distributions of moth flight directions were significantly different between naturally oriented and 180°-rotated night sky conditions, as well as significantly different between spring and autumn for any single sky condition (Fig. 3c–f), a Mardia–Watson–Wheeler test49 (Oriana) was used (Extended Data Fig. 2g).
Outdoor behaviour
The statistical procedures used for data collected outdoors were the same as those used for data collected indoors, with the exception that a likelihood-ratio test52 was used to test whether mean orientation directions for moth populations flown under two different clear natural sky conditions (earlier and later in the evening) were significantly different from each other (Fig. 2a,b). We did this by fitting maximum-likelihood distributions to the mean orientation directions for each trial, using the circular statistics package circular53 in R v.3.6.1 (www.r-project.org). In a similar manner, we determined the likelihood that the moth population flown under overcast conditions (Fig. 2c) had the same mean direction as either of the two populations flown under clear skies, in this case testing whether the means of the two different populations differed significantly.
Moreover, for the cohort of 95 moths flown under clear skies earlier and later in the evening (Fig. 2a,b), we used the mean orientation direction of each moth flown earlier (Fig. 2a), subtracted from the mean direction for the same moth flown later (Fig. 2b), and tested whether the mean angular differences were significantly different from zero (no change in heading). As the moth cohort was clearly less oriented later in the evening (possibly due to fatigue), we made this analysis by separating moths into three groups (Extended Data Fig. 2): (1) those that were well-oriented throughout the night (r > 0.8, both earlier and later in the evening, 35 out of 95 moths); (2) those that were well-oriented in only one trial (typically r > 0.8 earlier and r < 0.8 later, 42 out of 95 moths), and those that were less well-oriented throughout the evening (0.2 < r < 0.8 in both trials, 18 out of 95 moths). This analysis revealed that moths that were well oriented throughout the night (group 1) showed no change in heading, while those that were less-well oriented (groups 2 and 3) showed a westward drift later in the evening (Extended Data Fig. 2). However, this drift was not sufficient (in either direction or directedness) to significantly alter the mean direction of the population as a whole from earlier to later in the evening (Fig. 2a,b).
Electrophysiology
Spike train data were analysed using custom-written code in MATLAB (v.2019a and 2022b, MathWorks). Circular statistical analysis was performed in R (v.3.6.1; www.r-project.org) using the circular maximum-likelihood estimation package (CircMLE54). Responses were classified as unimodal (models M2A, M2B or M2C in the R-library CircMLE) or bimodal (models M4A or M4B) based on the Akaike information criterion. Only responses that were classified as unimodal (models M2A, M2B or M2C in the R-library CircMLE, based on the Akaike information criterion) were analysed further with respect to the half-width of the rotation tuning curve, the SNR and the variability of the response.
For cells that responded to night sky rotation (rotation angle φ) with an increase in spiking activity (which became maximal at the sky rotation angle φmax), the bootstrapped interquartile range of the unimodal part of the response distribution was taken to be an estimator of the half-width of the rotation tuning curve (φ range for 50% maximal response or greater). For cells that responded with a decrease in spiking activity, von Mises and mixed-von Mises distributions did not appropriately reflect this inhibition. Instead, the responses of these neurons were binned at 1° intervals and low-pass filtered, and the width at half-maximum was then determined in MATLAB (v.2019a and 2022b, MathWorks). The signal-to-noise ratio SNR was calculated in MATLAB as the ratio between the maximum response during night sky rotation (which occurs at a rotation angle φ = φmax), and the s.e.m. before the night sky rotation started. This was calculated for each stimulus rotation separately. To obtain an estimate of the reliability of a given cell’s response to repeated rotations of the night sky, the mean preferred stimulus rotation angle (φmax) was calculated as the circular mean for each individual night sky rotation. We then used the circular s.d.55 of the mean angle across all rotations as an estimate for the variability in the response.
Statistics and reproducibility
Behavioural data under natural clear starry skies (Fig. 2a,b) were obtained in independent experiments over three separate nights (with new moths used each night). Behavioural data under natural overcast skies (Fig. 2c) were obtained during only a single night. Behavioural data under projected starry skies generated using Stellarium (Fig. 3c–f) were obtained in independent experiments run over several nights with each night’s cohort of moths used only once. These experiments were repeated with the same results over two separate spring and autumn seasons (2018 and 2019). Thus, all of these experiments deal with biological replicates. Sample sizes were based on the availability of moths, with at least 40 moths (and as many as 70) being used for each experiment. Experiments were conducted without blinding and without a specific randomization protocol (apart from the random selection of moths used as replicates). Intracellular electrophysiological recordings (Fig. 4) and dye injections (Fig. 5) were obtained from single visual cells in the brain that were subjected to rotations of a projected starry sky (generated using Stellarium). Since each recording (and subsequent dye injection) was a unique observation, replication was not possible. This is due to the stochastic nature of intracellular recordings—single neurons were penetrated randomly from target brain regions that contain many thousands of cells.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.