Materials
The materials used were as follows: lead iodide (PbI2) (>98%, TCI Materials), methylammonium iodide (MAI) (Greatcell Solar), formamidinium iodide (FAI) (Greatcell Solar), NaOAc (Sigma-Aldrich), NaI (Sigma-Aldrich), H3PO2 (Sigma-Aldrich), EA (Sigma-Aldrich), ethanol (EtOH) (96%, Solveco), spiro-OMeTAD (99.5%) (Xi’an p-OLED), methylammonium chloride (MACl) (Xi’an Polymer Light Technology Corp.), caesium iodide (CsI) (99.999% Sigma-Aldrich), anhydrous N,N-dimethylformamide (99.8%, Sigma-Aldrich), dimethyl sulfoxide (DMSO) (99.9%, Sigma-Aldrich) and n-octylammonium iodide (OAI) (Greatcell Solar).
Preparation of aqueous solution for perovskite recycling
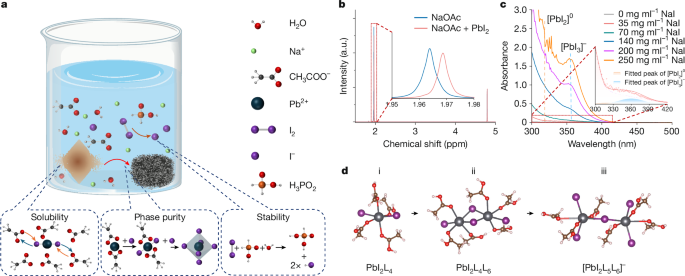
Three additives (NaOAc, NaI and H3PO2) were dissolved in deionized (DI) water, assisted by sonication with concentrations of 500 mg ml−1 for NaOAc, 800 mg ml−1 for NaI and 40 µl ml−1 for H3PO2. For example, 2.5 g of NaOAc was dissolved in 5 ml of DI water, followed by dissolving 4 g of NaI and the addition of 200 µl of H3PO2, to prepare the recycling solution for MAPbI3, FAPbI3 and mixed-cation perovskites. Note that the concentration for NaOAc, NaI and H3PO2 can also be varied in the ranges 100–700 mg ml−1, 300–900 mg ml−1 and 0.1–10.0 v/v%, respectively, to achieve the recycling of perovskite crystals.
Recycling of the perovskite
Each end-of-life perovskite module was soaked in the 20 ml aqueous solution at 80 °C for 20 min. After the perovskite layer was dissolved, the remaining part (SnO2-coated ITO substrate) was rinsed and removed from the solution. The solution was gradually cooled down at a rate of roughly 10 °C per hour to grow the perovskite crystals. The solid crystal and liquid solution were then separated by centrifuging at 5,000 rpm for 3 min. The obtained crystals were then washed with a mixture of ethanol (70%) and EA (30%) for three times and dried in a vacuum oven at 60 °C for 24 h to eliminate the residue. The effective drying process ensures that the recycling solvent of water does not pose a stability issue for the devices.
Recycling of spiro-OMeTAD
The de-encapsulated 20 modules were soaked in 10 ml of EA for 3 min. Afterwards, each module was rinsed with 5 ml of EA. Then, tetrabutylammonium iodide (8 mg) was introduced into the EA solution (total volume 110 ml). The solution was sonicated at 50 °C for 5 min, during which time it gradually transitioned from brown to light yellow. Following sonication, the solution was passed through a 0.5-cm-thick silica gel pad for filtration. Subsequently, the filtrate was rotary evaporated under reduced pressure until it reached a final volume of 1 ml. To this concentrated solution, 10 ml of 95% ethanol was added, resulting in a large amount of white solid precipitating from the solution. This suspension was stored in a refrigerator at 4 °C for 20 min and then centrifuged at 4,000 rpm for 3 min. The supernatant was carefully decanted and the white solid at the bottom was washed once with ethanol. Next, the white solid was dried in a vacuum oven at 50 °C for 2 h.
Recycling of the electrode
The degraded solar cells were collected and placed on the hotplate (preheated to 150 °C) for 3 min to soften EVA encapsulant and the cover glasses were then delaminated. After spiro-OMeTAD was dissolved with EA, the brown solution (about 110 ml) was centrifuged at 4,000 rpm for 5 min to collect the bottom-electrode powder. The electrode powder was washed twice with 10 ml of ethanol by sonicating for 10 min, centrifuging at 4,000 rpm for 5 min for each washing process and finally dried in a vacuum oven at 60 °C for 2 h. The collected electrode powder was directly added to the evaporation boat for recycled device fabrication without further treatment. The recycling ratio of the electrode was determined from the weight of the recycled electrode powder and the calculated amount of the electrode on the device from thickness (80 nm), device area and electrode density. The measured recycling ratio was 96.8%, which provided experimental data for life-cycle inventory (LCI) development.
Recycling of the SnO2 + ITO substrate
After perovskite recycling, the ITO + SnO2 substrates were sonicated in the 10 ml water/ethanol (50%/50% volume ratio) mixture for 15 min to remove the residue. Then the substrates were dried with a compressed nitrogen flow and treated with ultraviolet–ozone for 15 min.
Holistic recycling and multi-round recycling
The holistic recycling was conducted by a layer-by-layer recycling for the cover glass, electrode, spiro-OMeTAD, perovskite and SnO2 + ITO substrate as described above. The multi-round recycling was conducted by a repeated degradation–recycling process. The unencapsulated fresh devices were exposed to an accelerated degradation process at 85 °C with a relative humidity of 60% under AM 1.5G conditions until the device efficiency loss exceeded 20%. The end-of-life devices (about 400 substrates, including approximately 2,400 working pixels fabricated with the same composition) were holistically recycled as described above to fabricate the recycled devices. During the refabrication of recycled devices, the residue solution (for both spiro-OMeTAD and perovskite layer) on the wall of the spin coater was collected and reused to improve the utilization ratio. The recycled devices were degraded under the accelerated condition as before (85 °C with a relative humidity of 60% under AM 1.5G conditions) and then recycled. This procedure was repeated until the fifth-round recycled devices were completed. As the utilization ratio of the evaporation process is relatively low (<10%), extra electrode materials (100 mg in total for five rounds of recycling) were added to the recycled electrode to ensure a sufficient amount of materials for device refabrication. It further highlighted the importance of improving utilization ratios in device fabrication.
Precursor preparation
The control perovskite FA0.97Cs0.03PbI3 active layer precursor was prepared by dissolving 1.85 M lead iodide, 1.65 M FAI, 0.58 M MACl and 0.05 M CsI in 1 ml DMF and DMSO mixed solution at a volume ratio of 8:1. The recycled perovskite precursor was prepared by dissolving 1.65 M recycled FAPbI3 powder, 0.2 M PbI2, 0.58 M MACl and 0.05 M CsI in 1 ml DMF and DMSO mixed solvent (volume ratio 8:1).
The ion-modulated radical doping of spiro-OMeTAD was prepared as previously reported by mixing 90 mg ml−1 spiro-OMeTAD in chlorobenzene solution with 5 mol% spiro-OMeTAD2·+(TFSI−)2 and 8 mol% EDMPA+TFSI− (ref. 34).
Device fabrication
All ITO substrates were cleaned sequentially in DI water and ethanol for 15 min, respectively, and dried by a compressed nitrogen gun. After 15 min of ultraviolet–ozone surface treatment, the SnO2 electron transport layer was deposited by spin-coating a 1:6 diluted SnO2 nanoparticle aqueous solution (Alfa Aesar) at 4,000 rpm for 30 s, followed by annealing at 150 °C for 20 min in air. The perovskite layer was then deposited by means of spin-coating the perovskite precursor at 5,000 rpm for 30 s. Within 10 s of the 5,000-rpm spinning, 100 µl of chlorobenzene as the antisolvent was dropped onto the film. After spin-coating, the perovskite film was then annealed at 150 °C for 15 min in ambient air. Then 5 mg ml−1 OAI solution in IPA was spin-coated onto the perovskite surface at 5,000 rpm and annealed at 100 °C for 3 min for the surface passivation. Subsequently, the hole transport layers were deposited by spin-coating at 5,000 rpm for 30 s without further annealing. A metal electrode (80-nm Au) was finally deposited through the thermal evaporation method under a vacuum degree higher than 3 × 10−6 Torr to accomplish the solar cell fabrication. We used a 0.06-cm2 shadow mask to define the effective working area of the solar cells.
Device stability measurements
The recycled and fresh devices for stability measurements were fabricated following the same recipe. They were placed in an ageing box for one-sun light soaking in N2 or thermal stressed in an oven set to 85 °C in ambient air following the suggested procedure35.
DFT calculations
We carried out DFT calculations using the VASP code with projector augmented-wave potentials36,37,38. We used a plane-wave energy cutoff of 500 eV and a single Γ k-point for the molecular calculations. The exchange-correlation interactions were treated with the generalized gradient approximation of the Perdew–Burke–Ernzerhof (PBE) parametrization39. Grimme’s D3 correction was also included to deal with the van der Waals interactions40. We used a cubic box with length 20 Å for molecular calculations and a 8 × 8 × 5 k-mesh for the calculation of the PbI2 unit cell. The reaction equation for sodium iodine addition is Pb2I4L6 + I− → [Pb2I5L6]−.
LCA modelling
In our LCA work, we have methodically assessed various environmental impacts such as cumulative energy demand, carbon emissions and a comprehensive range of effects using the PEF approach32,41,42,43,44,45,46,47,48,49. The system boundaries were carefully defined to encompass the stages of raw material acquisition, module production and their subsequent recycling. The functional unit for our analysis is defined as one square metre of module area, aligning with industry standards for solar panel life-cycle analysis, despite not mirroring the dimensions of commercially available solar panels50,51.
We have compiled an exhaustive LCI dataset, which covers detailed material and energy flows at every stage of the life cycle for the perovskite solar cell under study (Supplementary Tables 1–12). In this work, copper is considered as a replacement for noble metals (gold or silver) to ensure that our assessment reflects future industry-relevant production processes, as has been done in the previous LCA report41. The calculations for material and energy use are based on experimental data from the fabrication and recycling processes (including both the utilization ratio and the recycling ratio of materials according to the literature42) of these perovskite solar cells. All equipment involved in the fabrication and recycling of perovskite solar cells operates on electricity, which is factored into the life cycle inventory43,52. The electrical consumption for each process is estimated by multiplying the power use by the operation duration throughout the solar cell fabrication and recycling stages. Although this approach may lead to an overestimation of the energy required for fabricating and recycling solar cells, it remains appropriate for this assessment. Given the substantial life-cycle environmental impacts embedded in materials compared with energy use, this method effectively demonstrates the environmental benefits of material retention through recycling as outlined in this study. Furthermore, for processes projected to scale up to industrial production, our estimations for energy consumption and material use are extrapolated from the existing literature, ensuring relevance and applicability53.
During the evaluation phase of our life-cycle impact assessment (LCIA), we have categorized the results according to various impact metrics, as per the chosen LCIA methodology. We used the PEF to reveal a comprehensive environmental profile of the perovskite solar cell being analysed. The LCIA data for the perovskite solar cell provided valuable insights into how the materials used and the processes involved contribute to different environmental impact indicators. The variations in sustainability metrics, according to the recycling frequencies, were analysed to align with specific environmental load lifetimes. These analyses emphasize the importance of considering both lifespan and recycling times when assessing the economic efficiency of perovskite solar cells.
Levelized cost of electricity
The LCOE is defined as the ratio of the total lifetime cost to the lifetime electricity production as follows42,54,55: \({\rm{L}}{\rm{C}}{\rm{O}}{\rm{E}}=\frac{{\rm{C}}{\rm{I}}+{\sum }_{t=0}^{N}\frac{{\rm{O}}{\rm{M}}(t,d)}{{(1+r)}^{t}}}{{\sum }_{t=0}^{N}\frac{E(t,d)}{{(1+r)}^{t}}}\). In the economic analysis of perovskite solar cells, CI denotes the upfront capital required to deploy the system, encompassing expenses related to the perovskite modules, installation labour, balance of system, inverters and permits, among other factors. OM denotes the annual operation and maintenance and module rejuvenation costs in year t. E stands for the annual electric power generated by the system in year t. N describes the expected service life of the PV system, whereas d indicates the rate at which the modules degrade each year. The discount rate, which is used to adjust future costs to the present value, is represented by r, following the precedent set by the existing literature55,56,57. These calculations take into account costs that are directly tied to the size of the system, the power output and a set initial investment for each project.
Recycling cost estimation
To estimate the cost of the recycled module, we aggregate various expenses, including utilities, labour, depreciation, maintenance and materials. Details about equipment costs, electricity consumption, process throughput and labour expenses are derived from the existing literature, facilitating our techno-economic analysis58,59,60 (Supplementary Table 10). Maintenance costs for the facilities are projected to be around 20% of the annual depreciation of the equipment. Finally, material costs are estimated by combining insights from the existing literature with quotes from material providers.