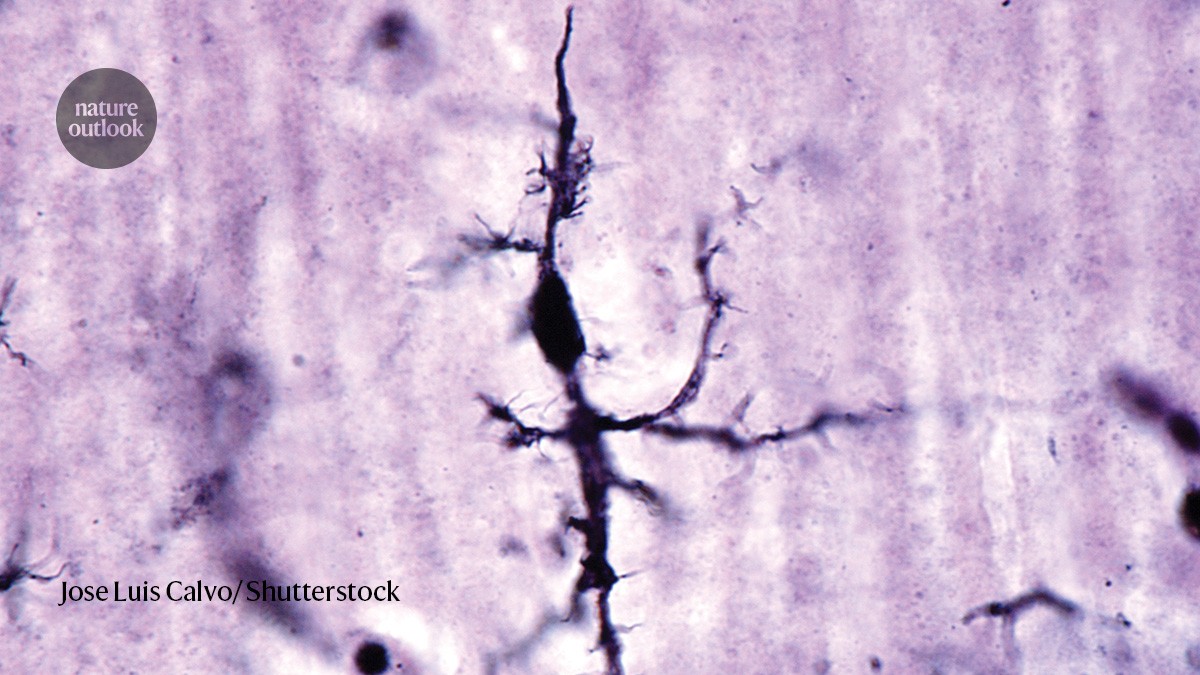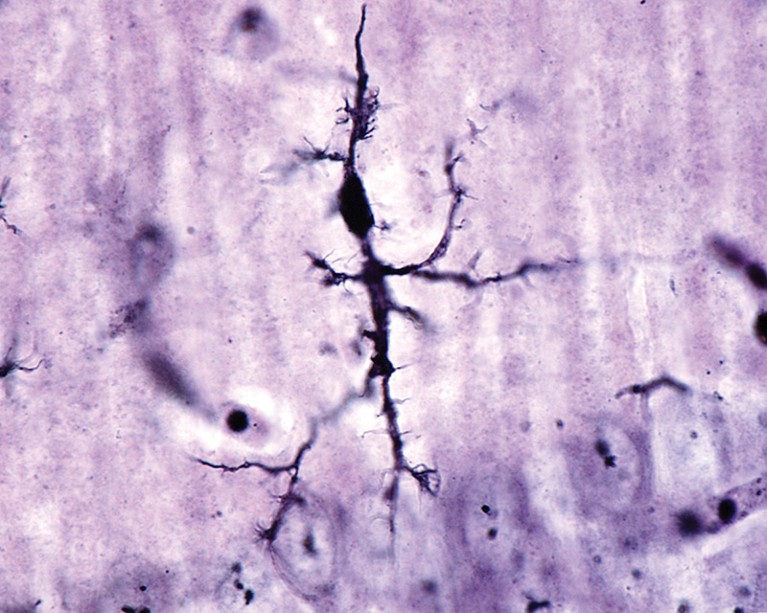
Microglial cell stained with a silver carbonate method.Credit: Jose Luis Calvo/ Shutterstock
‘Dark’ cells accelerate disease pathology
A type of immune cell called ‘dark microglia’ could contribute to neurodegeneration by producing lipids that damage neurons, say researchers led by Pinar Ayata at the City University of New York.
Microglia have various functions in the brain, including clearing debris and regulating the number of nerve junctions called synapses. Typically, they protect the brain, but in certain states they are thought to propel Alzheimer’s disease forwards.
Nature Outlook: Alzheimer’s disease
Ayata and her colleagues showed that the brains of people with Alzheimer’s contain twice as many microglia with a dark phenotype as do the brains of healthy adults. Dark microglia had previously been linked to neurodegeneration, but it was unclear how they entered this state.
The team says that the key is the integrated stress response (ISR) pathway, which is activated by the accumulation of the protein tau in people with the disease. By engineering mouse models of Alzheimer’s in which the ISR in microglia could be either activated or suppressed, the team showed that boosting the ISR increased the number of dark microglia and accelerated disease pathology, including the loss of synaptic connections between neurons. Dampening the ISR pathway had the opposite effect.
Having found that ISR activation promoted long-chain lipid production, the researchers proposed that dark microglia might broadcast their stress response to neighbouring neurons by releasing toxic lipids. They showed that bathing neurons in the medium in which dark microglia had been cultured produced harmful effects that could be eliminated by removing lipids from the medium. Drugs that inhibit the lipid-synthesizing enzyme also reversed synaptic degradation in mice, suggesting that this process could become a target for brain-protecting therapies.
Diabetes drug delivers Alzheimer’s upside
Semaglutide (Ozempic) was approved for treating type 2 diabetes in 2017, but has since been used as a weight-loss drug. Now, an analysis of Alzheimer’s diagnoses in people who were prescribed semaglutide or other medications for type 2 diabetes examines whether cases of Alzheimer’s disease emerged at different rates.
A group led by Rong Xu at Case Western Reserve University in Cleveland, Ohio, examined the health records of nearly 1.1 million US residents who had been prescribed medicines for type 2 diabetes and asked whether the rate of Alzheimer’s differed between people prescribed semaglutide and those given one of six other diabetes medications.
Diagnoses of Alzheimer’s were much less frequent in the roughly 17,000 people given semaglutide, which mimics the natural hormone glucagon-like peptide-1 (GLP-1). After three years of treatment, around one in 600 people taking semaglutide had been diagnosed with Alzheimer’s — roughly 70% less than in those prescribed insulin, and 40% lower than in people given another drug that activates GLP-1 receptors.
The protective effect seemed slightly stronger in women than in men, but it was equal in people with and without obesity. This is consistent with preclinical findings indicating that semaglutide protects against Alzheimer’s-related pathology. Clinical trials are now under way to assess whether the drug slows disease progression in people in the early stages of Alzheimer’s.
Alzheimers Dement. 20, 8661–8672 (2024)
A mechanistic link between hearing loss and dementia
Hearing loss is increasingly recognized as a risk factor for Alzheimer’s disease, but it has been unclear why. Researchers at Wuhan University in China found that a signalling molecule called growth differentiation factor 1 (GDF1) is depleted in the brains of mice with hearing loss — a change that might mediate Alzheimer’s-like pathology.
Zhentao Zhang and his colleagues focused on the hippocampus — a part of the brain that is crucial for forming memories and is affected by Alzheimer’s. In young mice, inducing deafness caused synapses in the hippocampus to be lost and reduced performance in two maze-based memory tests. In mouse models of Alzheimer’s disease, hearing loss worsened the rodents’ already declining cognitive abilities.
In both sets of mice, the team saw that expression of the gene GDF1 was strongly decreased in animals with hearing loss. Restoring normal GDF1 expression in the hippocampus prevented memory deficits in both sets of mice.
The researchers also showed that overexpressing GDF1 in mouse models of Alzheimer’s reduced the accumulation of the disease-associated protein amyloid-β in the brain. Conversely, blocking hippocampal expression of GDF1 in mice with intact hearing was sufficient to induce memory problems and increase amyloid-β deposition.
The study linked GDF1 to downstream pathways that have previously been implicated in the disease. The authors found that GDF1 levels are reduced in the brains of people with Alzheimer’s. The work suggests that drugs that restore GDF1-related functions could protect against Alzheimer’s.
Nature Aging 4, 568–583 (2024)
Five subtypes of Alzheimer’s identified
Proteins in the cerebrospinal fluid (CSF) of people with Alzheimer’s disease support dividing the condition into five distinct subtypes, each of which is dominated by different underlying disease processes.
Betty Tijms and her colleagues at the Free University of Amsterdam quantified more than 3,000 CSF proteins in 419 people with Alzheimer’s and 187 age-matched individuals without the disease. They found 1,058 proteins with different levels between the two groups. These were then used to categorize people with Alzheimer’s into five subtypes according to their protein levels. By analysing the functions of the proteins that were most affected in each subtype, the team inferred the dominant pathological process in each group. The most common ‘hyperplasticity’ subtype, accounting for 32.7% of cases, was marked by increases in proteins that contribute to synapse assembly and the generation of new brain cells. The next most frequent subtype, found in 29.6% of cases, involved increases in proteins that were specific to microglia, the brain’s immune cells, suggesting that these cells are overactive in this group.
In 18.6% of cases, there were increases in proteins involved in the functioning of the choroid plexus, a structure that is essential in CSF production. And 13.4% of individuals had increased levels of proteins implicated in defects of the blood–brain barrier, a protective membrane separating the two compartments.
A subgroup of only 5.7% of people was dominated by increases in proteins with functions that suggest potential changes in how neurons process RNA.
Different Alzheimer’s-associated risk genes were associated with each subtype to varying degrees, but no variant was exclusive to a single cluster. Furthermore, certain subtypes had distinguishing clinical features. For example, people in the groups associated with overactive microglia or decreased CSF production experienced more widespread shrinkage of brain tissue than did others.
These subtypes, the authors suggest, could benefit from targeted therapeutic approaches. They also propose that the subtype of participants in clinical trials be recorded to allow researchers to identify differences in how the groups respond to experimental treatments.
Metabolic enzyme reveals potential therapeutic target
In people with Alzheimer’s disease, the brain experiences a sustained decrease in the metabolism of glucose, an essential fuel. Researchers led by Katrin Andreasson at Stanford University in California have shown that inhibiting an enzyme known as indoleamine-2,3-dioxygenase 1 (IDO1) could reverse this metabolic deficit, potentially creating a path to restore brain function.
Metabolic changes in Alzheimer’s have been linked previously to problems with brain cells known as astrocytes. These cells metabolize glucose into lactate, which is then exported to neurons for use as fuel. However, astrocytes also contain the IDO1 enzyme, which converts the amino acid tryptophan to kynurenine, a metabolite which suppresses lactate production by the cells.
Andreasson and her colleagues have now shown that the proteins amyloid-β and tau — hallmarks of Alzheimer’s disease — can upregulate IDO1 activity in astrocytes, leading to an accumulation of kynurenine that decreases the conversion of glucose to lactate.
In a mouse model of Alzheimer’s (in which the animals have elevated kynurenine levels in the hippocampus), a drug that inhibits IDO1 reversed decreases in hippocampal glucose consumption and boosted spatial memory. The researchers also demonstrated that, in astrocytes generated from stem cells taken from people with Alzheimer’s, inhibiting IDO1 helped to restore the cells’ lactate production.
IDO1 inhibitors are already being developed for the treatment of cancer. The study suggests that using such drugs to prevent astrocytes producing excessive kynurenine could improve brain function by mitigating the effects of amyloid-β and tau deposits, rather than preventing their accumulation.
Randomized controlled trial shows benefits of lifestyle changes
A randomized controlled trial of intensive lifestyle changes for people with mild cognitive impairment and early Alzheimer’s disease has returned positive results. People who adopted a vegan diet, exercised and engaged in relaxation and psychological support activities for 20 weeks showed improvements in cognition, whereas those who made no lifestyle changes saw their symptoms worsen.
More from Nature Outlooks
Diabetes, obesity and physical inactivity are among several modifiable risk factors for Alzheimer’s. Trials in ageing populations have previously shown that lifestyle interventions can delay the onset of the disease, but this multicentre trial, led by Dean Ornish at the Preventive Medicine Research Institute in Sausalito, California, is the first to involve people who are in the early stages of Alzheimer’s.
People who undertook lifestyle changes were supplied with vegan meals and supplements, personalized exercise programmes, yoga-based stress-management interventions and access to psychological support. The control group, by contrast, received only usual care. All participants were assessed on four commonly used dementia tests before and after the 20-week period.
People who made no lifestyle changes scored worse on all four tests at the end of the study period. By contrast, those who received the interventions improved on three tests and maintained their performance on the fourth. Closer adherence to the lifestyle changes yielded better cognitive results. Furthermore, a blood biomarker of Alzheimer’s improved in people who changed their lifestyle, but worsened in those who did not.
The study involved only around 50 people, and blinding participants to which group they were in was not possible. Nevertheless, the authors argue that the results support larger, longer-term investigations of lifestyle interventions, potentially in combination with pharmaceutical therapies.