Human genetics assessment
Population cohorts
We included 453,072 participants in the UK Biobank, 164,353 participants in Geisinger Health System MyCode cohort, 41,251 participants in the Penn Medicine Biobank (UPENN-PMBB), 28,935 participants in the Malmö Diet and Cancer Study, 26,035 participants in the Mount Sinai BioMe Biobank and 4,736 participants recruited from Indiana University School of Medicine32,33. Systolic and diastolic BP readings were obtained from participants in the UK Biobank, Geisinger Health System MyCode cohort, UPENN-PMBB and the Malmö Diet and Cancer Study. In case of more than one BP reading, we used the median value. To correct for treatment with anti-hypertensive medication, 15âmmâHg was added to systolic BP readings and 10âmmâHg to diastolic BP readings in individuals with a history of treatment with anti-hypertensive medication, as previously done34,35. NT-proBNP was measured in the UK Biobank using a proteomics assay Olink36; in the Geisinger Health System MyCode cohort, measurements were extracted from electronic health records. HF was defined on the basis of International Classification of Diseases codes and procedure codes obtained from electronic health records as summarized in Supplementary Table 6.
DNA sequencing and genotyping data
The Regeneron Genetics Center performed high-coverage whole-exome sequencing using NimbleGen VCRome probes (Roche) or a modified version of the xGen design from Integrated DNA Technologies (IDT). Sequencing was performed using the Illumina v4 HiSeq 2500 or NovaSeq instrument, achieving over 20Ã coverage for 96% of VCRome samples and 99% of IDT samples. Variants were annotated using snpEff and Ensembl v85 gene definitions, prioritizing protein-coding transcripts on the basis of functional impact. The following variants were defined as protein truncating: insertions or deletions resulting in frameshift, any variant causing a stop gained, start lost or stop lost and any variants affecting a splice acceptor or splice donor site. Common-variant genotyping was performed on one of three single-nucleotide polymorphism array types as previously described: Illumina OmniExpress Exome array, Applied Biosystems UK BiLEVE Axiom Array or Applied Biosystems UK Biobank Axiom Array. We retained genotyped variants with a minor allele frequency (MAF) of >1%, <10% missingness and HardyâWeinberg equilibrium test Pâ>â10â15. We imputed the genotyped variants using the TOPMed reference panel37 using the TOPMed imputation server38,39. In GHS, imputation was performed separately by genotyping platform. Further details are provided elsewhere32,33,40,41.
Association analyses
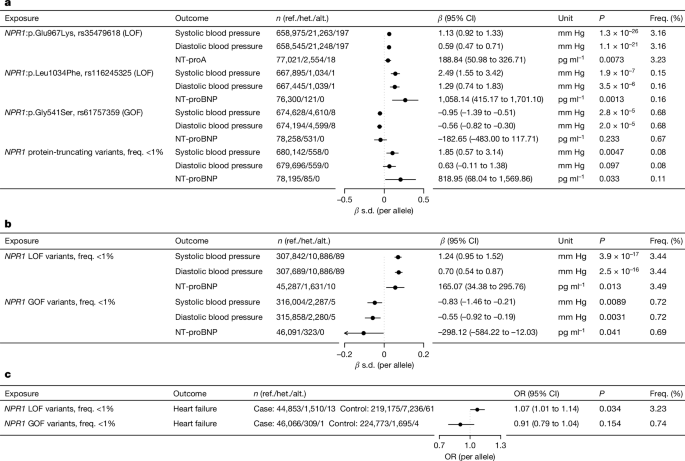
We estimated associations between protein-altering variants in NPR1 and phenotypes by fitting additive genetic, linear regression models (for quantitative traits; BP and NT-proBNP) or Firth bias-corrected logistic regression models (for binary traits; HF) using REGENIE (v.2+) software42. Analyses were stratified according to ancestry and were adjusted for age, age squared, sex, age-by-sex and age squared-by-sex interaction terms; experimental batch-related covariates; the first 10 common-variantâderived genetic principal components; the first 20 rare-variant-derived principal components; and a polygenic score generated by REGENIE, which robustly adjusts for relatedness and population structure42. Association results were meta-analysed across cohorts and ancestries using fixed-effects inverse variance weighting.
In addition to analysing individual protein-altering variants in NPR1, we evaluated two sets of gene-based burden genotypes: (1) a collection of rare predicted LOF variants; and (2) collections that include rare missense variants that are predicted to be GOF or LOF on the basis of experimental evidence or their association with BP. To characterize GOF or LOF, we first tested for association of rare and low-frequency (MAFâ<â1%), protein-truncating variants (presumed LOF) in NPR1 with BP and NT-proBNP across all available samples. Second, after exclusion of individuals with HF, we randomly split the cohorts with available BP readings in half into training sets (half of the samples) and test sets (half of the samples). All protein-altering variants in NPR1 with MAFâ<â1% and a minor allele count of â¥10 associated with increased systolic or diastolic BP (Pâ<â0.05) in the training set were characterized as presumed LOF, whereas variants associated with decreased systolic or diastolic BP in the training set were characterized as presumed GOF. The three previously published LOF or GOF variants15 were included regardless of these variant selection criteria. We then tested for association of the burden of presumed GOF and LOF variants with BP in the test sets and with NT-proBNP and HF in the combined set of test set samples and HF samples.
Generation of REGN5381
Fully human monoclonal antibodies against NPR1 were generated using VelocImmune technology21,22. The VelocImmune platform uses mice that are genetically modified to produce antibodies with human variable regions and enables efficient selection and development of fully human antibodies. Mice were immunized by the co-injections of human NPR1 and mouse ANP-expressing DNA plasmids, boosted with a protein complex of ANPâhNPR1. From the high-titre mice, splenocytes were isolated, and clones producing antibodies with desirable properties were identified and cloned to produce recombinant antibody proteins.
Surface plasmon resonance binding
The kinetics of REGN5381 NPR1-specific monoclonal antibody binding to NPR1 proteins were determined on the Biacore 8K, Biacore 4000, T200 or S200 biosensor (Cytiva), using the Series S CM4 sensor chip in filtered and degassed HEPES-buffered saline running buffer (10âmM HEPES, 150âmM NaCl, 3âmM EDTA, 0.05% (v/v) polysorbate 20, pHâ7.4) at 25â°C or 37â°C. The CM4 sensor chip was immobilized with mouse anti-human fragment crystallizable region (Fc) monoclonal antibody (REGN2567) or mouse anti-His monoclonal antibody (Cytiva) using standard amine-coupling chemistry43. REGN5381 NPR1 monoclonal antibodies were captured (100 RU-685 RU) through their Fc regions on anti-human Fc immobilized surfaces and varying concentrations of extracellular domains of NPR1, NPR2 and NPR3 proteins (with C-terminal MycMycHis) and ANP were injected followed by a dissociation phase. At the end of each cycle, anti-human-Fc-captured NPR1-specific monoclonal antibodies were removed using a 12âs injection of 20âmmolâlâ1 phosphoric acid.
All of the specific surface plasmon resonance-binding sensorgrams were double-reference-subtracted as reported previously44, and the kinetic parameters were obtained by globally fitting the double-reference-subtracted data to a 1:1 binding model with mass transport limitation using Biacore Insight Evaluation software (Cytiva). The dissociation rate constant (kd) was determined by fitting the change in the binding response during the dissociation phase, and the association rate constant (ka) was determined by globally fitting analyte binding at different concentrations. The equilibrium dissociation constant (KD) was calculated from the ratio of kd and ka. The dissociative half-life in minutes was calculated as ln2/(kdâÃâ60).
Cell binding and NPR1 bioassays
HEK293 cell lines were generated to stably express hNPR1 (amino acids M1 to G1061 of NP_000897.3) with C-terminal MYC and Flag tags or mfNPR1 (amino acids M1 to G1061 of XP_005541809.1). Flow cytometry experiments were performed with antibodies with or without 100ânM of human ANP or BNP (CytoFLEX flow cytometer) and the results were analysed using FlowJo. To assess NPR1 activity, bovine CNGA2 (amino acids Met1 to Pro663 of UniProt Q03041) was expressed along with NPR1. CNGA2 is a cation channel that can be activated by cGMP and can therefore be used as a readout for cGMP production23. NPR1 activation was monitored by measuring calcium influx through CNGA2 using a fluorescent Ca2+ indicator (Invitrogen Fluo-4 Direct Calcium Assay Kit; FLIPR TETRA, Molecular Devices). The numeric Ca2+ flux signal (one read per s, 700 total), expressed in relative fluorescence units, was calculated as the area under the time-signal-intensity curve from which the background signal observed before the addition of test reagents was subtracted. NPR1 activity was also evaluated by cGMP production (Cisbio cGMP kit, 62GM2PEH) with REGN5381 in the absence or presence of ligand at either 37â°C with 20,000 cells or room temperature with 1,000 cells. HEK293 cells have been authenticated via Human 16-Marker STR profile (IDEXX BioAnalytics) to be >80% identical to HEK293 cells sourced from ATCC, and tested negative for mycoplasma.
Physical complex assessment
A4F-MALS was used to assess the relative size distribution of complexes formed between REGN5381, hNPR1 ectodomain (REGN3037) and ANP. The A4F-MALS system is composed of a Wyatt Eclipse instrument and channel coupled to an Agilent 1260 Series High Performance Liquid Chromatography system equipped with an ultraviolet (UV) diode array detector, Wyatt Technology DAWN laser light-scattering (LS) instrument and an Optilab T-rEX differential refractometer (RI) detector. The detectors were connected in series in the following order: UVâLSâRI. The detector flow was maintained at 1.0âmlâminâ1 for the duration of the run, and the samples were injected onto the channel at 0.2âmlâminâ1 and were focused for 3âmin using a focus flow rate of 1.5âmlâminâ1. The separation step consisted of a linear gradient of the crossflow from 2.0âmlâminâ1 to 0.0âmlâminâ1 over 45âmin before holding the crossflow for a further 5âmin at 0.0âmlâminâ1 to allow any large particles to elute. The spacer in the channel was 350âW (Wyatt, 350âà in height) while the membrane was 10âkDa MWCO regenerated cellulose (Wyatt). The mobile phase for all experiments was 10âmM sodium phosphate and 500âmM NaCl pHâ7.0â±â0.1.
Cryo-EM analysis
Sample preparation and data collection
The NPR1 soluble domain was mixed with molar excess of the Fab fragment of REGN5381 in the presence or absence of ANP. The complex was incubated overnight at 4â°C then purified over the Superdex 200 increase 10/300 GL gel-filtration column equilibrated with 50âmM Tris pHâ7.5 and 150âmM NaCl. Peak fractions containing the NPR1-REGN5381 Fab complex were collected and concentrated using a 10âkDa molecular weight cut-off centrifugal concentrator (Amicon). For cryo-EM grid preparation, the protein sample was diluted to 1.6âmgâmlâ1 and 0.15% poly(maleic anhydride-alt-1-decene)-C8 amphipol was added. Then, 3âµl of protein was deposited onto a freshly plasma cleaned UltrAufoil grid (0.6/1, 300 mesh) using the Gatan Solarus II (Gatan) system. Excess solution was blotted away using filter paper and plunge-frozen into liquid ethane using the Vitrobot Mark IV (Thermo Fisher Scientific). The cryo-EM grid was transferred to a Titan Krios (Thermo Fisher Scientific) operated at 300âkV and equipped with a K3 detector (Gatan). Automated data collection was carried out using EPU (Thermo Fisher Scientific) at a nominal magnification of Ã105,000, corresponding to a pixel size of 0.86âà . A dose rate of 15 eââpxâ1âsâ1 was used and each video was 2âs, corresponding to a total dose of around 40âeââà â2. The defocus ranged from â1.4 to â2.4âµm.
NPR1âREGN5381 FabâANP complex
All cryo-EM data processing was performed using cryoSPARC (v.3.2.0)45. A total of 6,717 videos were collected; after alignment using patch motion correction followed by patch contrast transfer function estimation, 6,313 videos were selected for particle picking. An initial set of particles picked using a blob picker were subjected to 2D classification to generate templates for template picking (Extended Data Fig. 4aâc). In total, around 1.16âmillion particles picked by template picking were subjected to multiple rounds of 2D classification, yielding around 1.04âmillion particles in the optimal classes. Ab initio reconstruction with six classes generated different states of the NPR1 dimer with the REGN5381 Fab; the highest-resolution class had 682,951 particles corresponding to an NPR1 dimer with two REGN5381 Fabs and ANP bound. Non-uniform refinement of the particles in this class, followed by local refinement, resulted in a 3.1-Ã -resolution (Fourier shell correlationâ=â0.143) map that was used for model building. Into this map, we manually placed models of NPR1 dimer in complex with ANP (PDB: 1T34) and two Fabs (derived from other Regeneron antibody structures). These models were then manually rebuilt using Coot46, and real-space refined against the map using Phenix47. Cryo-EM data and model statistics are provided in Extended Data Table 1.
NPR1âREGN5381 Fab complex (no ANP)
For this complex, a total of 6,541 videos were collected; after alignment using patch motion correction followed by patch contrast transfer function estimation, 6,324 videos were selected for particle picking. An initial set of particles picked using a blob picker were subjected to 2D classification to generate templates for template picking (Extended Data Fig. 4dâf). In total, around 1.3âmillion particles picked by template picking were subjected to multiple rounds of 2D classification, yielding about 0.96 million particles in the optimal classes. Ab initio reconstruction with three classes generated different states of the NPR1 dimer with the REGN5381 Fab; the highest resolution class had 495,382 particles corresponding to an NPR1 dimer with two REGN5381 Fabs and ANP bound. Non-uniform refinement of the particles in this class, followed by local refinement, resulted in a 3.65-à -resolution (Fourier shell correlationâ=â0.143) map. This dataset has substantial preferred particle orientation, and the map resolution is anisotropic and, in many areas, a good deal worse than 3.65âà (Extended Data Fig. 4d). Although this map clearly defined the overall conformation of the NPR1âREGN5381 complex, and showed no density at the ANP-binding site (Extended Data Fig. 4d), it was not feasible to carry out model building and refinement in the manner that we did for the NPR1âREGN5381âANP complex. Instead, that complex model was split into two half-complex models, each containing one NPR1 ectodomain and one REGN5381 Fab. These half-complexes were rigid-body refined against the NPR1âREGN5381 (no ANP) map using Phenix47, producing the model shown in Fig. 2. Cryo-EM data and model statistics are provided in Extended Data Table 1.
Preclinical in vivo mouse studies
NPR1
hu/hu mice
NPR1hu/hu mice were generated on the C57BL/6NTac (75%)/129S6SvEvTac (25%) background using the Velocigene platform48. The generation of these mice was necessitated by the poor conservation of the NPR1 amino acid sequence between human and mouse, and the need to generate a human antibody. Each animal was implanted with PA-C10 (Data Sciences) radiotelemetry devices for the recording of central arterial pressures. The transmitter was located in the carotid artery of each mouse.
All of the mouse experiments were conducted in compliance with protocols approved by the Regeneron Pharmaceuticals Institutional Animal Care and Use Committee, in accordance with state and federal guidelines. Mice were fed normal chow (PicoLab Rodent Diet 20, 5001), housed under standard conditions and allowed to acclimatize for at least 7âdays before being placed on the study.
REGN5381 was diluted with sterile phosphate-buffered saline for subcutaneous injection into mice.
REGN5381 single-dose mouse study
Telemetered NPR1hu/hu mice were randomly assigned to one of five dosing groups (nâ=â4â6 per group) on the basis of body weight, and received a single subcutaneous dose of saline or REGN5381 (1, 5, 25 or 50âmg per kg) on study day 0. The BP of each animal was continuously monitored for 28âdays. Urine samples for urinalysis and urinary biomarkers were collected on day 28 after dosing.
REGN5381 versus standard-of-care therapies
Comparisons of REGN5381 alone and in combination with standard-of-care therapies were conducted as a series of four separate experiments. For each experiment, telemetered NPR1hu/hu mice were stratified by systolic BP into one of four dosing groups (nâ=â5â7 per group). The groups then received a single subcutaneous dose of saline or REGN5381 (25âmg per kg), immediately followed by an oral gavage dose of water or standard-of-care therapy (enalapril 25âmg per kg per day; valsartan 125âmg per kg per day; sacubitril 120âmg per kg per day; sildenafil 40âmg per kg per day; milrinone 5âmg per kg per day; propranolol 5âmg per kg per day; nifedipine 20âmg per kg per day; sacubitril/valsartan 100âmg per kg; empagliflozin 30âmg per kg; spironolactone 100âmg per kg). Oral dosing continued once daily in the morning for 7âdays. The dose volume for each animal was based on the most recent body weight measurement. Each animalâs BP was continuously monitored for 7âdays.
Ex vivo vessel reactivity
NPR1hu/hu mice were euthanized by CO2 inhalation followed by laparotomy. The abdominal aorta, third-order mesenteric arteries and mesenteric vein were dissected. Arterial and vein segments (~2âmm in length) were placed into ice-cold Krebs buffer solution and prepared for wire myography. Vascular rings were mounted onto wires in the chambers of a multivessel myograph (J P Trading) filled with Krebs buffer maintained at 37â°C (refs. 49,50,51). The buffer was gassed with 95% O2â5% CO2. After equilibration for 30â60âmin, the vessels were set to an internal circumference equivalent to 90% of what they would be in vivo when relaxed under an intraluminal pressure of 100 mmâHg (ref. 49). Isometric tension was monitored continuously before and after experimental interventions and was expressed as mNâmmâ1 vessel length. The experiments were initiated after a 30â60âmin stabilization interval. In each vessel, the constrictor response to 80âmmolâlâ1 KCl was determined. Vessels were precontracted with phenylephrine (10âÃâ10â6âmolâlâ1), and the vasodilatory response to acetylcholine or test article was measured. Data were expressed as meanâ±âs.e.m. Concentrationâresponse data derived from each vessel was fitted separately to a logistic function by nonlinear regression, and the maximum asymptote of the curve (maximal response) and concentration of agonist producing EC50 were calculated using commercially available software (GraphPad Prism v.9.0.2). Concentrationâresponse data were analysed using two-way analysis of variance followed by Tukeyâs test. All other data were analysed using one-way analysis of variance or the Studentâs t-tests for unpaired samples.
Non-human primate pharmacodynamic study
Thirty cynomolgus monkeys (Macaca fascicularis) of Mauritius origin, 2â4 years of age and weighing 3â5âkg (Charles River Laboratories), were enrolled in the study. Each animal was implanted with PhysioTel Digital model L11 (Data Sciences International) radiotelemetry devices for recording the central arterial pressure and ECG waveforms. The transmitter was located intramuscularly in a dorsal position and lateral to the median plane below the ribs, and the attached BP catheter was located in the femoral artery with the tip of the catheter located in the descending aorta.
The animals were acclimatized to laboratory housing for a minimum of 5âweeks before initiation of dosing (including recovery time after surgical implantation when performed). During treatment, non-human primates were housed individually in stainless-steel cages in a controlled environment under standard conditions. They were provided Purina Certified Primate Diet 5048 biscuits twice daily and assorted fresh fruit or vegetables. Reverse-osmosis-filtered water was provided ad libitum by means of an automatic watering system. All non-human primate experiments were conducted in compliance with protocols approved by the Charles River Institutional Animal Care and Use Committee, in accordance with state and federal guidelines.
Telemetered animals were randomly assigned to one of six dosing groups (nâ=â5 per group) and received a single subcutaneous dose of saline or REGN5381 (1, 5 or 25âmg per kg bolus), or a single intravenous dose of REGN5381 (5 or 25âmg per kg bolus) on study day 1. Each animalâs BP was continuously monitored for 28 days, and thereafter once weekly for 24âh until the completion of the study on day 56. Urine samples for urinalysis and urinary biomarkers were collected from a cage pan in the morning at baseline and 24âh after dosing. Blood samples for clinical chemistry and the determination of systemic REGN5381 concentrations in the serum were collected from all animals before dosing, and on days 2, 3, 4, 7, 14, 28, 42 and 56 after dosing. Blood samples collected at predose and on day 56 were also analysed for ADA. Concentrations of total REGN5381 (all drugs; without regard to binding site occupancy) were determined using an enzyme-linked immunosorbent assay. ADA analysis was conducted using a generic immunoassay against anti-human IgG4 antibodies.
Canine telemetry study
Two beagle canines (Canis lupus familiaris) 8â10 months of age and weighing 9â10.5âkg were received from Marshall BioResources and enrolled in the study. For telemetry studies, each animal was implanted with PhysioTel Digital model D70 (Data Sciences) radiotelemetry devices for recording the central arterial pressure.
Animals were acclimatized to the laboratory housing for a minimum of 2âweeks before initiation of dosing (including recovery time after surgical implantation when performed). During treatment, canines were housed in groups of two in a controlled environment under standard conditions. They were provided PMI Nutrition international Certified Canine LabDiet 5007 daily. Reverse-osmosis-filtered water was provided ad libitum. All canine experiments were conducted in compliance with protocols approved by the Charles River Institutional Animal Care and Use Committee, in accordance with state and federal guidelines.
A cross-over design was used, and each canine received a single intravenous bolus dose of saline (control, nâ=â2), followed by a 7-day washout period. Canines then received a single intravenous slow bolus of 25âmg per kg REGN5381 (nâ=â2). Radiotelemetry data were acquired continuously, beginning 48âh before the first administration of vehicle or REGN5381, and continued for 48âh after dosing.
Acute anaesthetized canine study
Twelve beagle canines (C. l. familiaris) 11â13 months of age and weighing 9â15âkg were received from Covance and enrolled in the study. General procedures for animal care and housing met current American Association for Accreditation of Laboratory Animal Care International recommendations, Guide for the Care and Use of Laboratory Animals and the US Department of Agriculture through the Animal Welfare Act (as amended, and conformed to testing facility SOP)52,53. Canines were housed in runs (up to two canines per run). The temperature and humidity ranges of the study room were set to maintain 74â±â10â°F and 50â±â20%, respectively. The light cycle was set to maintain 12âhâ12âh onâoff. Canines were offered Certified Canine Diet (LabDiet 5007) once daily except on the day of that canineâs scheduled experiment; each canine was kept on an overnight fast before the day of its scheduled experiment. Canines were provided with fresh water ad libitum using an automatic watering system except when removed from the run. Anaesthetized canines were instrumented with venous and arterial catheters. Each canine received a single intravenous bolus dose of saline (control, nâ=â6) or 25âmg per kg REGN5381 (nâ=â6). Following dose administration, canines were monitored for acute haemodynamic changes.
Before dose administration, each canine was anaesthetized and instrumented for cardiovascular data collection. After placement of all cardiovascular instrumentation, each canine had baseline haemodynamic parameters collected before dosing while they were anaesthetized. Each dose was administered as a single intravenous bolus to the animal. Dose volumes were based on each canineâs most recent individual body weight. After dose administration, the animals were monitored for acute haemodynamic changes. Throughout each experiment, blood and urine samples, as well as haemodynamic parameters, were collected.
Canine surgical catheter implantation
A minimum of two venous catheters were placed into a peripheral vessel (for example, cephalic and/or saphenous) for administration of anaesthetics and analgesics. General anaesthesia was induced intravenously (bolus) with propofol (approximately 6âmg per kg) and α-chloralose (30â100âmg per kg), aided by fentanyl (approximately 5âµg per kg). A cuffed endotracheal tube was placed and used to mechanically ventilate the lungs with up to 100% O2 through a volume-cycled animal ventilator (10â15 breaths per min with a tidal volume of 15â30âml per kg) to sustain end-tidal expired CO2 values within the normal physiological range (35â45âmmâHg). Anaesthesia was maintained by intravenous infusion of α-chloralose (20â55âmg per kg per h), with analgesia provided by intravenous infusion of fentanyl (2.0â10.0âµg per kg per h) to preserve normal autonomic function, as well as inotropic/lusitropic properties. Fentanyl and α-chloralose infusions ran concurrently through the same, single peripheral venous catheter. The second venous catheter was used for dosing of control/test article. Surface electrodes were placed onto the thorax for the recording of body-surface ECGs (for example, Lead II). The body temperature was maintained within the normal physiological range by temperature-controlled warming blankets.
Local anaesthesia (bupivacaine 0.5%, 1â2âml per site) was administered subcutaneously over each surgical site before surgical manipulation. Once a surgical plane of anaesthesia was achieved, a cut-down over a jugular vein and carotid artery was performed for placement of fluid-filled introducers. Two vessel introducers were secured within the jugular vein/jugular vein branches. Through one of the jugular access catheters, a flow-directed Swan Ganz sampling catheter was advanced into the pulmonary artery for the determination of pulmonary artery pressures, right atrial pressures/CVPs. Through the carotid artery, a catheter was advanced into the left ventricle for assessment of left ventricular pressure. A cut-down over the femoral triangle was used to access the femoral artery and vein. A fluid-filled introducer was secured within the femoral artery; through this femoral artery introducer, a solid-state pressure transducer was advanced into the artery to collect an arterial pressure waveform.
ANP and BNP expression constructs
The mouse BNP hydrodynamic delivery construct was constructed by ligating synthetic DNA constructs (Genscript) containing the Nppb coding sequences through the BglIIâNotI restriction sites into the vector pRG977-mRor-V5 (N-term.) to create the final constructs used for injection into mice. The reference sequence used in the design of the expression construct was mouse Nppb reference protein (NCBI: NP_032752). The mouse ANP hydrodynamic delivery construct was constructed by ligating synthetic DNA constructs (Genscript) containing the Nppa coding sequences into the vector pRG977 to create the final constructs used for injection into mice. The reference sequence used in the design of the expression construct was mouse Nppa reference protein (NP_032751). On study day 0, mice were stratified into groups on the basis of body weight. Mice were injected through the tail vein with 50âµg plasmid in sterile saline at 10% of body weight.
Clinical study
REGN5381 in a first-in-human study
A phase 1, double-blind, placebo-controlled, two-part single-ascending dose study was designed to assess the safety, tolerability and pharmacodynamics/pharmacokinetics of REGN5381 in healthy adults (18â55 years of age; NCT04506645). Data reported here include only findings from the first part, which was conducted in healthy adults (18â55 years of age) at a single site. The study protocol was approved by institutional review boards or relevant ethics committee, and the research ethics committee of the University Hospitals of Leuven. The study was conducted in accordance with the ethical principles originating from the Declaration of Helsinki, and was consistent with the International Conference on Harmonization/Good Clinical Practices and applicable regulatory requirements. All of the participants provided written informed consent.
Participants were screened for systolic BP 100â140âmmâHg (inclusive) and diastolic BP 60â90âmmâHg (inclusive). If the participants in a dosing cohort experienced clinically important hypotension or tachycardia, the BP inclusion criteria could be adapted to allow for increases in the range (systolic BP, 130â165âmmâHg; diastolic BP, 60â100âmmâHg), although adaptation of the inclusion criteria was not necessary. After initial screening, the participants attended a 2-day inpatient treatment/observation period before dosing and were placed on a fixed-sodium diet to ensure consistency in sodium intake and to reduce the variability in BP due to dietary sodium. Non-invasive haemodynamics were measured using pulse-wave analysis technology (ClearSight, Edwards Lifesciences) beginning 1 day before randomization and continued through to the end of the inpatient monitoring period.
The participants were randomized 6:2 to receive single-dose intravenous REGN5381 (0.3, 1, 3, 10, 30 or 100âmg) or intravenous placebo. After study drug administration on day 1, the participants remained in the clinic until day 4 to allow careful monitoring of haemodynamics. On day 4, the participants were assessed for safety before discharge. The study duration was based on predicted blood levels of REGN5381 concentrations in the serum across all dose cohorts; the end of the study visit was conducted no earlier than 21âdays after the administration of the study drug. The primary end point in this study was the type, incidence and severity of TEAEs after a single intravenous dose administration of REGN5381 or placebo over time.
Statistical analyses
Preclinical assessments
Statistical analysis was performed using standard statistical software (GraphPad Prism 7). Analysis of data were performed using one-way analysis of variance or two-way repeated-measures analysis of variance. Tukeyâs post hoc multiple-comparison test (αâ=â0.05) was used. Data are reported as meanâ±âs.e.m. Pâ<â0.05 was considered to be significant.
First-in-human clinical assessments
There was no formal primary efficacy analysis in this study. For continuous variables, descriptive statistics included the following information: the number of participants reflected in the calculation (n), mean, s.d., quartile 1, quartile 3, median, minimum and maximum. Plots of the values over time, as well as the change or percentage change over time, were provided.
Haemodynamic parameters are known to be sensitive to the nominal time of the day; for example, a participant may have a relatively higher BP during the daytime than at night. Thus, a time-matched change from baseline in systolic BP, diastolic BP, mean arterial pressure, pulse pressure and cardiac stroke volume across the first 24âh after dose was reported by treatment group. For example, the time-matched change from baseline at 07:00 was calculated as follows:
$${{\rm{Chg}}}_{07:00}={{\rm{BP}}}_{{\rm{07:00\; D1}}}-{{\rm{BP}}}_{{\rm{07:00\; D-1}}}$$
where BP07:00 D1 was the BP collected at 07:00 on day 1 and BP07:00 Dâ1 was the blood pressure collected at 07:00 on day â1. Time-matched mean change from the baseline for the pulse-wave analysis was provided by treatment group.
For categorical or ordinal data, frequencies and percentages were displayed for each category. Unless otherwise specified, the participants randomized to placebo were pooled across cohorts.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.