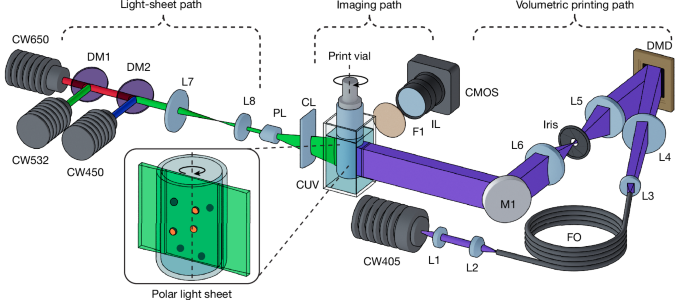
Tomographic volumetric printer
The custom-built volumetric printer used in this study (Fig. 1a) used a 405-nm laser source shaped into a flat-top intensity profile through coupling of the beam into a square core fibre (WF 70 × 70 μm, CeramOptec). This shaped beam was collimated and used to illuminate a DMD (Hi-Speed V-7000, ViALUX), for which the resultant projection was Fourier filtered and imaged with 1:1 magnification using a 4f relay onto the centre of the printing volume in a telecentric projection regime. During the printing process, vials were immersed within a refractive index compensating bath comprising a square water-filled quartz cuvette (OP36, eCuvettes). This was required to minimize refractive errors arising from the curvature of the vial surface, for both printing and feature scanning. To accommodate different print requirements, we used borosilicate glass vials (Readily3D) with internal diameters ranging from 8.8 to 15.3 mm, selected according to the specific model parameters and target print volumes. In terms of compatible resins, tomographic volumetric printing is compatible with processing light-crosslinkable materials, whereas other classes of materials can be added as inclusions in the resin vat, for instance, by means of embedded extrusion printing3, before polymerizing the resin.
Light-sheet imaging module
The light-sheet imaging path (Fig. 1) used three 40–50-mW diode lasers operating at 450 nm (RLDD450-40-5, Roithner), 532 nm (RLDD532-50-3, Roithner) and 650 nm (RLDH650M-40-5, Roithner) to cover a broad range of commonly used fluorophores. The beams were combined with dichroic mirrors, shaped into a flat-top fan profile with a 30° Powell lens (43-473, Edmund Optics) and then focused to the vial centre using an f = 50 mm cylindrical lens. Two scanning regimes were used: a half-sweep mode covering Θtotal = π (500 polar sections) and a Θtotal = 2π sweep mode (1,000 polar sections). The latter was preferentially used for samples exhibiting occlusive or highly scattering samples, as this provided bidirectional illumination of the features. Image acquisition for feature scanning/registration was performed using a monochromatic camera (Alvium 1800 U-240m, Allied Vision) in conjunction with an f = 50 mm C-Mount lens (MVL50M23, Thorlabs). With this hardware, we achieved a resolution of 14.47 µm along the image plane, suitable for capturing cellular aggregates and organoids. Higher-resolution hardware would be needed to extend the detection to the single-cell regime, hence the current system is limited to detecting larger particles. Exposure and gain parameters were manually set before each scanning session. A rotary filter mount containing band-pass filters for several commonly used fluorophores (GFP, Cy3.5, Cy5) was integrated post-objective to facilitate spectral selection of the emission signal while rejecting the backscattered laser line. Notably, although light-sheet imaging has been historically an expensive technology, new reports describe how to build open-source, affordable light-sheet systems. In our case, our simplified set-up required only the use of a laser source, a beam reducer, a Powell lens for beam shaping and a cylindrical lens for focusing the light sheet within the volume—all available as off-the-shelf components.
Image processing and feature registration for GRACE
A MATLAB script was prepared to perform several functionalities key to the GRACE workflow. This process (shown in Extended Data Fig. 1a, and detailed extensively in Supplementary Methods 4) was responsible for the following: (1) the initialization and synchronization of hardware (imaging, light sheet and printing) and software parameters; (2) the acquisition and processing of polar light-sheet image stacks; (3) the isolation and registration of features of interest from the background; (4) the conversion of image data to usable 3D coordinate data; (5) the processing of these data (for example, using clustering detection) to extract construct-specific information necessary for generating the desired construct; and (6) outputting these data for use with the parametric modelling software.
Data-driven parametric models
We used off-the-shelf software Rhino3D (Robert McNeel & Associates) in conjunction with its integrated Grasshopper (GH) visual programming environment. GH definitions were developed to create parametric models for each geometry type. These definitions performed three key tasks: (1) importing and synchronizing with data files containing coordinates, radii and other relevant data as described above; (2) using these data to generate the desired parametric geometry; and (3) baking and exporting the final model as an STL file suitable for 3D printing. Our work focused on three broad types of biologically inspired geometry: perfusable vessel-like channels surrounding scanned features with an inlet and an outlet, positive interconnected geometries and targeted single-layer encapsulation. In several of these cases, although not strictly necessary, we also used two freely available add-ons for GH. These were the Dendro plug-in42, which facilitated convenient surface generation around point structures and the ShortestWalk plug-in43, which makes use of the A* algorithm41 to calculate the shortest walk in a network of paths. We note that similar functionalities can be provided using other plug-ins (either inbuilt or third party) or by writing a custom script within GH. See Supplementary Methods 8 for further details on the creation of the parametric models and definitions.
Synthesis of GelMA
All chemicals were obtained from MilliporeSigma and used without further purification or modification, unless stated otherwise. GelMA was synthesized as previously reported44. Briefly, 0.6 g of methacrylic anhydride were added per gram of gelatin (type A, from porcine skin, 10 w/v% in phosphate-buffered saline (PBS)) and left to react for 1 h at 50 °C under constant stirring, to obtain a degree of methacryloyl substitution of 80%, as assessed through 1H-proton nuclear magnetic resonance (NMR; 400 MHz, Agilent 400-MR NMR, Agilent Technologies). The resulting solution was dialysed (MW cut-off = 12 kDa) against deionized water to remove the unreacted methacrylic anhydride. The purified macromer solution was sterile filtered (0.22 µm), freeze-dried and stored at −20 °C until used.
Resin preparation and printing
GelMA-based bioresin supplemented with 0.1% w/v LAP (Tokyo Chemical Industry) photoinitiator was used for experiments in this work. Before printing, resins were thermally gelled by immersing in ice water for 10 min. Schlieren imaging was used to observe the crosslinking process during printing, for which the process was manually stopped on observing a sufficient accumulation of dosage, as evidenced by a rapid change in the refractive index of the resin. After printing, samples were washed with warm (37 °C) PBS to remove uncrosslinked material. The mechanical characterization of the printed hydrogels is reported in Supplementary Methods 20 and Supplementary Fig. 17.
Linear light-sheet scanning of samples post-printing
For all experiments in this paper, printed samples were imaged using a custom-built, linearly swept light-sheet fluorescence microscope. Samples were placed in a 22 × 22-mm square cuvette (OP36, eCuvettes) and immersed in PBS during imaging. Sections were captured at 35-μm steps over the span of the entire sample.
Using GRACE to detect and print around alginate particles
For all work involving alginate microparticles (microparticle preparation is described in Supplementary Methods 6), resins were prepared with 10% GelMA w/v + 0.1% LAP w/v. The stained alginate particles were laden into the volume and gently suspended by agitating the volume while being cooled in an ice bath until the resin thermally gelled. These were then scanned over Θtotal = π and cluster detection was used to determine the centroid coordinates of each alginate sphere (as described above in the pipeline for feature coordinate registration in GRACE). An example of detecting and keeping track (through selective illumination) of a particle is depicted in Supplementary Video 3. For prints involving two populations of stained alginate particles, this process was performed twice to obtain a separate coordinate dataset for each group. The corresponding adaptive models were then generated and printed according to the GRACE workflow. This approach could also be performed in the presence of alginate capsules, each loaded with a concentration of 200 million cells per ml (Supplementary Fig. 18, Supplementary Information Methods 21 and Supplementary Video 4).
Auto-alignment of sequential prints
Auto-alignment of sequential prints was accomplished using a combination of GH and MATLAB. In our work, this was demonstrated with a femur and cartilage model, although the same workflow can be performed to automatically align any two or more geometries sequentially. The process began by importing a reference femur model into GH, in which a section of geometry from the femoral head was extracted and assigned an adjustable extruded thickness, allowing for customizable cartilage thickness. The relative positioning between the two models defined the target alignment between the sequential geometries a priori. To accomplish this alignment practically, we sought to determine the rigid transformation required to align the reference femur (and thus the relatively placed cartilage model) to the location of the arbitrarily located scanned femur within the vial. First, a stained femur (Cy5) was printed using 10% GelMA w/v + 0.1% LAP w/v, washed and resuspended into new GelMA resin (stained with Cy3.5) at a random orientation. This print was then scanned, and the resulting volumetric point cloud data Pscan = {q1, q2,…, qm} was exported. A dense array of random points PRef = {p1, p2,…, pn} was then generated within the volume of the reference femur model and then also exported to MATLAB. The reference femur point cloud (Pref) was automatically aligned to the scanned femur (Pscan) using the iterative closest point (ICP) algorithm (MATLAB’s ‘pcregistericp’ function). This generated a rigid transformation matrix T, such that, when also applied to the reference cartilage geometry GRef, resulted in a rotation and translation of the cartilage component to its correctly aligned position over the scanned femoral head within the vial, such that G′Aligned = TGRef. The transformation data were synchronized to the GH definition, thus generating a correctly placed cartilage geometry that was subsequently exported and printed. By using any reference models and assigning correct relative positions within GH, this process can be used for any arbitrary alignment task, providing a versatile approach for complex, multistage printing processes that require precise spatial relationships between sequentially printed components. Notably, once the reference geometries and relative positions were pre-established, the entire alignment process could be accomplished <15 s after scanning. Additional information on auto-alignment and overprinting applications is included in Supplementary Methods 22 and 23.
Shadow correction of pillar occlusions
An occluding structure comprising ten parallel pillars 0.5 mm in diameter attached to a base was fabricated using a Formlabs Form 3B+ stereolithography (SLA) printer with opaque grey resin (Formlabs Gray Resin V4) (Fig. 3c, Supplementary Fig. 10a and Supplementary Methods 11). The occluder was placed into vials and filled with 1 ml of 10% GelMA w/v + 0.1% LAP w/v. The GRACE workflow was performed as previously described but without emission filters during scanning. Instead, the reflected and scattered laser line was imaged over a Θtotal = 2π sweep, enabling approximate surface reconstruction (Supplementary Fig. 10b) by using the light sheet as a profilometer. Cluster detection was used on the surface data to identify the centroids and a principal component analysis determined the pitch and yaw of the pillars (Supplementary Fig. 10c and Supplementary Methods 11 for details). A parametric model was prepared in GH to generate the representative occludsion to overlay with the scanned data at the correct angles and locations. This 3D model of the occlusion, along with a symmetrical cog model, was exported for processing using the OSMO algorithm21 to create an optimized set of shadow-corrected projections for printing. It should be noted that the shadow correction algorithm is not designed to prevent or mitigate artefacts caused by (unwillingly) introducing bubbles within the resin vat. As such, careful handling and gentle pipetting or pouring of the resin is always recommended when loading the printable materials into the vials. Moreover, the efficacy of the corrective algorithm reduces proportionally to the number and size of occluding elements present in each plane within the print volume, as evidenced by the analysis reported in Supplementary Fig. 9. Because the exact number, shape and size of occluding elements that can be tolerated for printing are not constant and depend on the architecture to be printed, it is advisable to perform in silico simulations, as reported in Methods, ahead of printing experiments, to evaluate printability.
Shadow correction for ball-in-cage model
A spherical cage-like occluding structure was fabricated using stereolithography (Fig. 3e), following the same protocol and material as with the occluding pillars. The geometry was algorithmically generated in GH through a three-step process: (1) 45 nodes were randomly distributed on the surface of a 10-mm-diameter sphere; (2) for each node, paths were computed to its five nearest neighbours based on spatial proximity; (3) struts of 0.5 mm diameter were generated along these paths, connecting each node to its five nearest neighbours. This procedure yielded a complex, non-uniform, interconnected spherical cage structure (Supplementary Fig. 11a), presenting a challenging occlusion scenario for volumetric printing. The cage was embedded within the resin, thermally gelled and scanned using the light sheet as a profilometer, as previously described. This scan generated a sparse point cloud representing the occluding surface (Supplementary Fig. 11b). In contrast to the pillar experiment, in which the complete occlusion geometry was reconstructed parametrically, here we instead used the auto-alignment protocol previously developed for the femur-cartilage model. A reference cage mesh was algorithmically aligned to the scan-derived point cloud, enabling the correctly oriented reference mesh to serve as the computational occlusion volume during projection optimization using the OSMO algorithm. A 5-mm-diameter sphere positioned within the cage served as the target geometry for optimization and volumetric printing. Post-printing, the corrected and uncorrected structures (printed in triplicate) were washed, removed from the occluding cages and imaged using linear light-sheet microscopy (Supplementary Fig. 11c). Prints were quantified on the basis of their r.m.s. error and sphericity (Fig. 3f–h; see Supplementary Methods 12 for further information). Moreover, using this same shadow correction and printing approach, a more complex trifurcated vascular network was printed within a hydrogel enclosed into a stent-like occluding mesh, to demonstrate the possibility to resolve also hollow and negative features (Supplementary Methods 13, Supplementary Fig. 12 and Supplementary Video 5).
iβ-cells subculture and expansion
iβ-cells, an engineered pancreatic cell line mimicking β-cell function and capable of releasing insulin together with the luminescent reporter NanoLuc, were obtained as previously described in the literature26,27. iβ-cells were cultured (95% humidified incubator at 37 °C, 5% CO2) in Roswell Park Memorial Institute (RPMI) 1640 Medium, containing GlutaMAX and HEPES (Gibco, Life Technologies) supplemented with foetal bovine serum (FBS, 10% v/v), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. For all experiments, cells were used at passages 3 and 4.
GRACE printing within constructs produced by embedded extrusion
A toroidal extrusion path of 4 mm in diameter was designed and saved as a G-code file. First, the volumetric printing vial was loaded with 5% GelMA at 37 °C + 0.1% w/v LAP. The vial was left to thermally gelate at room temperature overnight and then placed in a R-GEN 100 extrusion printer (RegenHU) and centrally retained using a custom bracket. The GelMA hydrogel was used as a photoreactive suspension bath for embedded extrusion printing. Extrusion printing was performed with the R-GEN 100 printer by means of a pneumatic-driven extrusion printhead. As bioink for extruding the toruses, iβ-cells (5.0 × 107 cells ml−1, stained with the Vybrant DiD membrane dye (ThermoFisher Scientific) to facilitate imaging) were suspended in a 2% w/v alginate solution, used as fugitive viscosity enhancer to improve printing resolution of the high-density cell suspension. The bioink was loaded into 3-ml cartridges equipped with a 23 G stainless steel straight, cylindrical needle (Nordson EFD). Toruses were then extrusion-printed in sterile conditions within the GelMA support bath. After this, the vial containing the support bath and the cell-laden bioink was transferred to the volumetric printer. Following the GRACE workflow, the samples were imaged by means of light sheet and a set of blood-vessel mimetic channel networks were parametrically generated by the software to wrap around the cell-laden toruses (Fig. 4a and Supplementary Methods 14). Using tomographic volumetric printing, the design was printed into the GelMA bath, forming the biological tissue construct. This produced tapered channel networks around the toruses with a 300-μm offset, fixed surface area of 180 ± 10 mm2, minimum diameter of 450 μm and an inlet and outlet of about 1 mm at both ends of a cylindrical bulk scaffold. After volumetrically printing these constructs, the vial was heated to 37 °C to dissolve the unpolymerized GelMA and the sample was retrieved and washed with prewarmed PBS. It should be noted that, although the CAD design of the extruded cell structures is known, it is still preferred to apply the GRACE algorithm after 3D imaging of the extruded features and not directly on the geometry from the CAD file. Generating the parametric design after imaging, in fact, ensures that any printing artefact, or loss of shape fidelity owing to the viscoelastic nature of the cell-based ink, is taken into account45 (Supplementary Fig. 19). Moreover, this also takes advantage of the ability of GRACE to auto-align the volumetric print onto the features of interest, reducing inaccuracies and errors that could be caused by the manual alignment of the print vial. Furthermore, two control groups were produced: (1) a bulk cylindrical structure containing no channels printed around the extruded toruses as negative control and (2) a set of randomly generated channels running along the length of the cylindrical bulk, also maintaining the same 180 ± 10 mm2 surface area, to provide a comparable interface for solute exchange from the printed vessels to the hydrogel. Equal surface areas of the random and GRACE-printed constructs were verified post-printing by using linear light sheet to scan the samples and then manually segmenting the channels and determining the surface areas. From these data, the average minimum torus to vessel distance and the average vessel diameter were calculated (Supplementary Fig. 13).
Analysis of embedded extrusion prints
The printed constructs (n = 6 for each group) with different generated vessel networks were retrieved and cultured overnight in incubator in the presence of RPMI 1640 medium, GlutaMAX, HEPES (Gibco, Life Technologies) supplemented with 10% v/v FBS and 1% penicillin/streptomycin. Dynamic culture was permitted by placing the samples on an orbital shaking platform (95 rpm), to ensure media displacement and flow. The following day, the supernatant was collected for each different condition and the bioluminescent reporter NanoLuc (directly related to the amount of insulin stored and released by iβ-cells) was quantified using the NanoLuc Luciferase Kit (Promega Corporation) against a standard curve, using a CLARIOStar Plus multimodal plate reader (BMG Labtech) (Fig. 4d).
Osteochondral differentiation in the cell-laden femur-cartilage model
At passage 4 (see Supplementary Methods 15 for cell isolation and expansion protocol), ACPCs and MSCs were encapsulated in 10% w/v GelMA solution in PBS at a final density of 1.0 × 107 cells ml−1 and 5.0 × 106 cells ml−1, respectively. LAP dissolved in PBS at 0.1% w/v was used as a photoinitiator for the crosslinking. To minimize scattering owing to the presence of cells, 30% v/v of iodixanol (OptiPrep) was used in the MSC-containing resin, whereas a 20% concentration was used for resin containing ACPCs. Vybrant DiD and DiO (ThermoFisher Scientific) were used as cell-labelling membrane staining for MSCs and ACPCs, respectively. Cells were stained for 30 min at 37 °C according to the manufacturer’s instructions. Bone-cartilage models were scanned, aligned and printed with GRACE (Fig. 4e), in the same fashion as described previously with acellular constructs. Post-printing, the cell-laden constructs containing both the bone and cartilage layers were washed in warm PBS to remove the unpolymerized biomaterial and cultured in 1:1 ratio osteogenic (Osteogenic Differentiation Medium BulletKit, PT-3002, Lonza) and chondrogenic media (DMEM, supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 0.2 mM L-ascorbic acid-2-phosphate, 1% v/v Insulin-Transferrin-Selenium (ITS) + Premix Universal Culture Supplement (Corning), 0.1 × 10−6 M dexamethasone and 10 ng ml−1 recombinant human TGF-β1) for 4 weeks. Media were refreshed three times a week.
Combining FLight with GRACE
Each stained population of spheroids (see Supplementary Methods 17 for MSC spheroids preparation protocol) was scanned using either an excitation wavelength of 450 nm (for DiO) or 650 nm (for DiD), in conjunction with the appropriate emission band-pass filter. The centroid coordinates of each spheroid population were determined and synchronized with a parametric model. This model was designed so as to position either a cylindrical or a star-shaped geometry centred at each spheroid, with DiO-stained features receiving circles and DiD receiving stars. Following model export and system homing, we used FLight fabrication (Fig. 4g,h,j). This involved projecting a single binary image of the geometry at its face-on angle into the stationary vial, with the rotational stage fixed at the corresponding angle of the projection. Illumination was maintained until crosslinking was observed through approximately 70% of the vial volume, as determined by real-time schlieren imaging. The uncrosslinked material was then washed in warm PBS, linear light sheet imaged in the vial and then collected and imaged under confocal microscopy (Fig. 4i).
Statistical analysis
Data are expressed as mean ± standard deviation (s.d.), with a minimum sample size of n ≥ 3. Statistical analyses for experiments involving shadow correction were conducted using OriginPro 8.5 (OriginLab) and Prism 9 software (GraphPad Software Inc.) was used for the EmVP experiments. Normal distribution was assumed. Pairwise comparisons between two groups were performed using a Student’s two-sample two-tailed t-test (α = 0.05), with statistical significance defined as P < 0.05. For analyses involving more than two groups, a one-way analysis of variance (ANOVA) was used with post hoc Turkey’s multiple comparison test.
Ethical statement
Animal tissue and cells used in this study were obtained from an existing, commercially available cell line, as described in Methods, or deceased equine donors, donated to science by their owner, and according to the guidelines of the Institutional Animal Ethical Committee of Utrecht University.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.